Notes: Phases of Matter
... C- Bernoulli’s Principle: as the velocity of a gas/liquid increases, the pressure it exerts decreases. 1- Plasma: is a special type of gas that is broken up into positively and negatively charged particles. Example neon in neon ...
... C- Bernoulli’s Principle: as the velocity of a gas/liquid increases, the pressure it exerts decreases. 1- Plasma: is a special type of gas that is broken up into positively and negatively charged particles. Example neon in neon ...
Black Hole Entropy in String Theory.
... entropy formula is modified. If there is a Killing horizon, then one can associate an entropy, Wald ...
... entropy formula is modified. If there is a Killing horizon, then one can associate an entropy, Wald ...
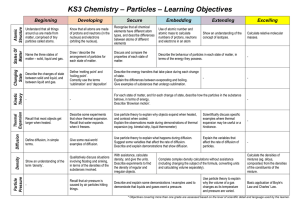
Phase Rule and Binary Phase Diagrams
... • System: The portion of the universe that is being studied • Surroundings: The part of the universe not included in the system ...
... • System: The portion of the universe that is being studied • Surroundings: The part of the universe not included in the system ...
Chemistry Chapter 11
... Take square roots: vA / vB = √MB / √MA Because rate of effusion is directly proportional to molecular velocity we can say ...
... Take square roots: vA / vB = √MB / √MA Because rate of effusion is directly proportional to molecular velocity we can say ...
Ideal Gas Law - ISMScience.org
... If 36.0 L of CO2 is produced during the above reaction, how much O2 was used? 58.5 L ...
... If 36.0 L of CO2 is produced during the above reaction, how much O2 was used? 58.5 L ...
Unit 8: Momentum, Impulse, and Collisions
... o In collisions of all kind, the initial and final total momenta are equal. In elastic collision between two bodies, the initial and final total kinetic energies are also equal and the initial and final relative velocities have the same magnitude. In an inelastic collision the total kinetic energy i ...
... o In collisions of all kind, the initial and final total momenta are equal. In elastic collision between two bodies, the initial and final total kinetic energies are also equal and the initial and final relative velocities have the same magnitude. In an inelastic collision the total kinetic energy i ...
Solutions - University of Illinois at Chicago
... (d) What are the limiting values of the entropy as T → 0 and as T → ∞ ? How would your results change if it was a spin-1/2 particle? In the limit T → 0 (or B → ∞ ), only the lowest (ground) state is occupied. The multiplicity of the ground state is Ω = 1 . Therefore, S = k B ln Ω → 0 In the limit T ...
... (d) What are the limiting values of the entropy as T → 0 and as T → ∞ ? How would your results change if it was a spin-1/2 particle? In the limit T → 0 (or B → ∞ ), only the lowest (ground) state is occupied. The multiplicity of the ground state is Ω = 1 . Therefore, S = k B ln Ω → 0 In the limit T ...
Theoretische Physik IV: Statistische Mechanik, Exercise 6
... (b) Using the result from (a) show that absolute zero cannot be reached by an adiabatic expansion. In the following we gain intuition whether absolute zero can be reached at all. We consider the fact that cooling processes always take place between two curves with X = const., e.g. X1 = P1 , X2 = P2 ...
... (b) Using the result from (a) show that absolute zero cannot be reached by an adiabatic expansion. In the following we gain intuition whether absolute zero can be reached at all. We consider the fact that cooling processes always take place between two curves with X = const., e.g. X1 = P1 , X2 = P2 ...
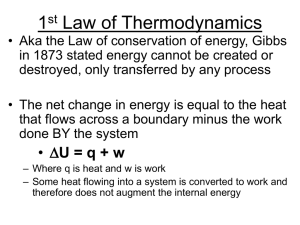
Lecture 5 - Thermodynamics II
... • Besides knowing volume changes, need to figure out how S changes with T For internal energy of a thing: dU = dqtot – PdV; determining this at constant volume dU = CVdT where CV is the heat required to raise T by 1°C ...
... • Besides knowing volume changes, need to figure out how S changes with T For internal energy of a thing: dU = dqtot – PdV; determining this at constant volume dU = CVdT where CV is the heat required to raise T by 1°C ...
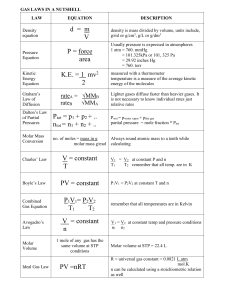
CHM 111: General Physical Chemistry 3 Units
... empirical gas laws, Ideal Gas Equation of State, qualitative treatment of kinetic theory of gases, real gases and deviations from ideal gas laws; liquid, macroscopic properties of liquids, evaporation, vapor pressure and its variation with temperature, boiling point, heat of vaporization, Clausius-C ...
... empirical gas laws, Ideal Gas Equation of State, qualitative treatment of kinetic theory of gases, real gases and deviations from ideal gas laws; liquid, macroscopic properties of liquids, evaporation, vapor pressure and its variation with temperature, boiling point, heat of vaporization, Clausius-C ...