un/scetdg/36/wpxx
... This instruction applies to UN 3XXX and UN 3YYY For cylinders, pressure drums and tubes, the general packing requirements of 4.1.6.1 shall be met. Unless otherwise indicated in these Regulations, cylinders, pressure drums and tubes conforming to: (a) The applicable requirements of Chapter 6.2; or (b ...
... This instruction applies to UN 3XXX and UN 3YYY For cylinders, pressure drums and tubes, the general packing requirements of 4.1.6.1 shall be met. Unless otherwise indicated in these Regulations, cylinders, pressure drums and tubes conforming to: (a) The applicable requirements of Chapter 6.2; or (b ...
General Chemistry Stoichiometry Notes
... Quantitative Analysis Composition stoichiometry- mass relationships of elements in compounds. ...
... Quantitative Analysis Composition stoichiometry- mass relationships of elements in compounds. ...
Thermochemistry
... Calorimetry is the accurate and precise measurement of heat change for chemical and physical processes. Calorimeters are devices used to measure the amount of heat absorbed or released during chemical and physical processes. Enthalpy is the heat content of a system ...
... Calorimetry is the accurate and precise measurement of heat change for chemical and physical processes. Calorimeters are devices used to measure the amount of heat absorbed or released during chemical and physical processes. Enthalpy is the heat content of a system ...
CD Roms - 香港道教聯合會圓玄學院第一中學
... 1. to recognize that protons, neutrons and electrons are constituents of the atom. 2. to balance nuclear reaction equations. 3. to recognize that the relative isotopic, atomic and molecular masses can be determined by mass spectrometer. 4. to understand the mole concept. ...
... 1. to recognize that protons, neutrons and electrons are constituents of the atom. 2. to balance nuclear reaction equations. 3. to recognize that the relative isotopic, atomic and molecular masses can be determined by mass spectrometer. 4. to understand the mole concept. ...
Screen Version
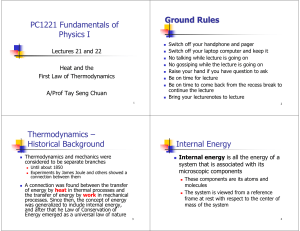
... An infinite warm reservoir of heat (H) at constant temperature T1, and an infinite cold reservoir for heat (C) at constant temperature T2 (where T1 > T2) are available. Also, an insulating stand S to facilitate adiabatic changes. Heat can be supplied from the warm reservoir to the working substance ...
... An infinite warm reservoir of heat (H) at constant temperature T1, and an infinite cold reservoir for heat (C) at constant temperature T2 (where T1 > T2) are available. Also, an insulating stand S to facilitate adiabatic changes. Heat can be supplied from the warm reservoir to the working substance ...
Engines and the Second Law of Thermodynamics
... Example 20-8: Heat transfer. A red-hot 2.00-kg piece of iron at temperature T1 = 880 K is thrown into a huge lake whose temperature is T2 = 280 K. Assume the lake is so large that its temperature rise is insignificant. Determine the change in entropy (a) of the iron and (b) of the surrounding enviro ...
... Example 20-8: Heat transfer. A red-hot 2.00-kg piece of iron at temperature T1 = 880 K is thrown into a huge lake whose temperature is T2 = 280 K. Assume the lake is so large that its temperature rise is insignificant. Determine the change in entropy (a) of the iron and (b) of the surrounding enviro ...
Topic 4 - Lloyd Crosby
... c. Group I A hydroxides and Group II A hydroxides (from Ca on) are strong electrolytes. d. Most other substances are nonelectrolytes. B. Nonelectrolytes 1. Definition A substance that does not ionize (does not produce any ions) when dissolved in water; a solution of a nonelectrolyte either does not ...
... c. Group I A hydroxides and Group II A hydroxides (from Ca on) are strong electrolytes. d. Most other substances are nonelectrolytes. B. Nonelectrolytes 1. Definition A substance that does not ionize (does not produce any ions) when dissolved in water; a solution of a nonelectrolyte either does not ...
chemistry
... 51 Draw a Lewis electron-dot diagram for an atom of silicon. [1] Base your answers to questions 52 through 54 on the information below. ...
... 51 Draw a Lewis electron-dot diagram for an atom of silicon. [1] Base your answers to questions 52 through 54 on the information below. ...
writing chemical equations
... In the previous single replacement reaction example, we have written the molecular equation for the reaction. Although this equation shows the reactants and products of the reaction, it does not give a very clear picture of what truly occurs in solution. In fact, such an aqueous solution actually co ...
... In the previous single replacement reaction example, we have written the molecular equation for the reaction. Although this equation shows the reactants and products of the reaction, it does not give a very clear picture of what truly occurs in solution. In fact, such an aqueous solution actually co ...
On the Importance of Prereactive Complexes in
... ruled out the formation of HC(O)OH + H but not the reaction ...
... ruled out the formation of HC(O)OH + H but not the reaction ...
Ocular emergencies2 - King Saud University Medical Student
... • Ocular pain, redness and discharge with decrease vision and white lesion on the cornea ...
... • Ocular pain, redness and discharge with decrease vision and white lesion on the cornea ...
enjoy learning - System Dynamics Society
... The paintings of impressionists often leave us a profound impression as follows: when taking a close look at them, we find those strokes, of which a piece of painting consists, only are numerous fine points of different colors. However, as we gradually stand back, we realize that, a certain change o ...
... The paintings of impressionists often leave us a profound impression as follows: when taking a close look at them, we find those strokes, of which a piece of painting consists, only are numerous fine points of different colors. However, as we gradually stand back, we realize that, a certain change o ...
SAMPLE AP CHEMISTRY EXAM QUESTIONS
... Ethanol is burned in oxygen. Solid barium oxide is added to distilled water. Chlorine gas is bubbled into a cold, dilute solution of potassium hydroxide. A solution of iron(II) nitrate is exposed to air for an extended period of time. Excess concentrated sulfuric acid is added to solid calcium phosp ...
... Ethanol is burned in oxygen. Solid barium oxide is added to distilled water. Chlorine gas is bubbled into a cold, dilute solution of potassium hydroxide. A solution of iron(II) nitrate is exposed to air for an extended period of time. Excess concentrated sulfuric acid is added to solid calcium phosp ...
Does electrical double layer formation lead to salt exclusion or to
... the natures of the charge-forming processes. In the ideal case, in order to apply an external potential difference between two metal electrodes, these interfaces should be polarizable, implying that the applied potential does not leak away by Faradaic currents 共there are no interfacial redox reactio ...
... the natures of the charge-forming processes. In the ideal case, in order to apply an external potential difference between two metal electrodes, these interfaces should be polarizable, implying that the applied potential does not leak away by Faradaic currents 共there are no interfacial redox reactio ...
Your views are welcomed upon the theme of
... outer shell or an octet of electrons in the outer shell. Helium has the former, but not the latter. Argon has the latter, but not a full outer shell. Only the atom of neon has both.) Discrete atoms that do not have this type of outer shell structure are seldom found in nature: so single atoms of car ...
... outer shell or an octet of electrons in the outer shell. Helium has the former, but not the latter. Argon has the latter, but not a full outer shell. Only the atom of neon has both.) Discrete atoms that do not have this type of outer shell structure are seldom found in nature: so single atoms of car ...
Exergy: the quality of energy
... Thermodynamics are based on experience, experience with nature that shows which conversions from one kind of energy into the other are possible and which are not. In the following several kinds of energy will play a role like: kinetic energy, potential energy, internal energy, heat, work, electrical ...
... Thermodynamics are based on experience, experience with nature that shows which conversions from one kind of energy into the other are possible and which are not. In the following several kinds of energy will play a role like: kinetic energy, potential energy, internal energy, heat, work, electrical ...
Chemistry 30 - SharpSchool
... a substance that appears to act as a Brønsted-Lowry acid in some rxns and a Brønsted-Lowry base in other rxns is said to be _____________ ...
... a substance that appears to act as a Brønsted-Lowry acid in some rxns and a Brønsted-Lowry base in other rxns is said to be _____________ ...
Supporting Text S1.
... positive. Therefore it is possible that (GSh GTu ) changes sign on increasing temperature, as shown schematically in Fig. S3a. The sign change of (GSh GTu ) upon increasing temperature implies the entropy change (SSh STu 0) . Another possible source of entropy change is through liberat ...
... positive. Therefore it is possible that (GSh GTu ) changes sign on increasing temperature, as shown schematically in Fig. S3a. The sign change of (GSh GTu ) upon increasing temperature implies the entropy change (SSh STu 0) . Another possible source of entropy change is through liberat ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.