June 01, 2008
... As a chemist working in the forensics department of the SAPS, you were called upon to identify an unknown powder that was found at 3 different crime scenes. Your preliminary examination of the powder samples obtained from Scene 1 and Scene 2 might suggest it being an illegal drug (a date-rape drug). ...
... As a chemist working in the forensics department of the SAPS, you were called upon to identify an unknown powder that was found at 3 different crime scenes. Your preliminary examination of the powder samples obtained from Scene 1 and Scene 2 might suggest it being an illegal drug (a date-rape drug). ...
February Homework Packet
... Matter can be a in the solid, liquid, or gas phase Chemistry is the study of the change in matter Physical changes are changes in matter in which the appearance of a substance changes but the identity of the compound remains the same Chemical changes are changes in matter in which the identi ...
... Matter can be a in the solid, liquid, or gas phase Chemistry is the study of the change in matter Physical changes are changes in matter in which the appearance of a substance changes but the identity of the compound remains the same Chemical changes are changes in matter in which the identi ...
Equilibrium
... Kp = 6.8 x 10-9 If COCl2(g) at an initial pressure of 1.00 atm decomposes, calculate the equilibrium pressures of all species? ...
... Kp = 6.8 x 10-9 If COCl2(g) at an initial pressure of 1.00 atm decomposes, calculate the equilibrium pressures of all species? ...
Chapters 13 and 14
... a. What does point V represent? What characteristics are specific to the system only at point V? b. What does each point on the curve between V and W represent? c. Describe the changes that the system undergoes as the temperature slowly increases from X to Y to Z at 1.0 atmosphere. d. In a solid-liq ...
... a. What does point V represent? What characteristics are specific to the system only at point V? b. What does each point on the curve between V and W represent? c. Describe the changes that the system undergoes as the temperature slowly increases from X to Y to Z at 1.0 atmosphere. d. In a solid-liq ...
Spring Exam 2 - Chemistry
... This is VERY IMPORTANT! Under IDENTIFICATION NUMBER, put in your 8 DIGIT STUDENT ID NUMBER (do not use the 9 at the beginning of your number) beginning in column A and continuing through column H, column I will be blank, (do NOT use column J at this time); be sure to fill in the correct circles (a c ...
... This is VERY IMPORTANT! Under IDENTIFICATION NUMBER, put in your 8 DIGIT STUDENT ID NUMBER (do not use the 9 at the beginning of your number) beginning in column A and continuing through column H, column I will be blank, (do NOT use column J at this time); be sure to fill in the correct circles (a c ...
2/22 Lecture Slides
... Next quiz will be from Homework Set 2 (after Exam 1) Lab reports (glassware resubmission – due 2/27; Cl lab report – due 3/8) ...
... Next quiz will be from Homework Set 2 (after Exam 1) Lab reports (glassware resubmission – due 2/27; Cl lab report – due 3/8) ...
First Law of Thermodynamics
... M is converted into heat when it strikes the floor.* We divide the surroundings into a * The kinetic energy of the system is transferred to the molecules in thermal and mechanical components. The thermal surroundings exchange energy with a the floor, which causes their temperature to rise. Cars are ...
... M is converted into heat when it strikes the floor.* We divide the surroundings into a * The kinetic energy of the system is transferred to the molecules in thermal and mechanical components. The thermal surroundings exchange energy with a the floor, which causes their temperature to rise. Cars are ...
Homework,1 Atoms, molecules, and ions
... 3- For the following reaction at equilibrium in a reaction vessel, which one of the changes below would cause the Br2 concentration to decrease? 2NOBr(g) 2NO(g) + Br2(g) ΔHorxn = 30 kJ a decrease the container volume c. Increase the temperature. ...
... 3- For the following reaction at equilibrium in a reaction vessel, which one of the changes below would cause the Br2 concentration to decrease? 2NOBr(g) 2NO(g) + Br2(g) ΔHorxn = 30 kJ a decrease the container volume c. Increase the temperature. ...
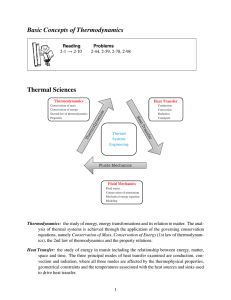
Basic Concepts of Thermodynamics Thermal Sciences
... two independent and intensive properties) • it is important to be able to: – find two appropriate properties to fix the state – find other properties when the state is fixed (we will discuss this later) ...
... two independent and intensive properties) • it is important to be able to: – find two appropriate properties to fix the state – find other properties when the state is fixed (we will discuss this later) ...
EGR 107 FALL 2001
... US2 Air in a piston cylinder undergoes a three process cycle. Initially, the air has a specific volume of 3cubic meters per kg and a pressure of 120kPa. The air is compressed and heat is removed for an isobaric process until the volume is reduced to one third of its original volume. Next the air is ...
... US2 Air in a piston cylinder undergoes a three process cycle. Initially, the air has a specific volume of 3cubic meters per kg and a pressure of 120kPa. The air is compressed and heat is removed for an isobaric process until the volume is reduced to one third of its original volume. Next the air is ...
Chemistry from Telephone Numbers: The False Isokinetic Relationship George C. McBane
... step of each oxidation should be similar. If that is the case, one might expect the enthalpy and entropy of activation ∆H ‡ and ∆S ‡ to change in a simple way as the chain length of the substrate is increased. The term isokinetic relationship was first defined as a linear dependence of ∆H ‡ on ∆S ‡ ...
... step of each oxidation should be similar. If that is the case, one might expect the enthalpy and entropy of activation ∆H ‡ and ∆S ‡ to change in a simple way as the chain length of the substrate is increased. The term isokinetic relationship was first defined as a linear dependence of ∆H ‡ on ∆S ‡ ...
Laboratory 3
... In this equation, the (+) symbol indicates that nitrogen reacts with oxygen and the arrow indicates that nitric oxide is formed. The chemical formulas on the left side of the equation are collectively known as the reactants and those on the right side as the products. In this case we have one kind o ...
... In this equation, the (+) symbol indicates that nitrogen reacts with oxygen and the arrow indicates that nitric oxide is formed. The chemical formulas on the left side of the equation are collectively known as the reactants and those on the right side as the products. In this case we have one kind o ...
Chapter 3: Stoichiometry
... as many entities as the number of atoms in exactly 12 grams of the 12C isotope of carbon. Avogadro’s number is the experimentally determined number of atoms in 12 g of isotopically pure 12C, and is equal to 6.022 x 1023 One mole of anything contains 6.022 x 1023 entities – 1 mol H = 6.022 x 1023 ato ...
... as many entities as the number of atoms in exactly 12 grams of the 12C isotope of carbon. Avogadro’s number is the experimentally determined number of atoms in 12 g of isotopically pure 12C, and is equal to 6.022 x 1023 One mole of anything contains 6.022 x 1023 entities – 1 mol H = 6.022 x 1023 ato ...
powerpoint
... 2. Not all particles have same KE, avg KE of particles=temp of gas 3. Elastic collisions between particlestransfer of energy w/ no loss(Total energy stays the same.) 4. Volume of gas particles ignored compared to volume of space in which they contain. 5. Gas particlesno attraction to each other ...
... 2. Not all particles have same KE, avg KE of particles=temp of gas 3. Elastic collisions between particlestransfer of energy w/ no loss(Total energy stays the same.) 4. Volume of gas particles ignored compared to volume of space in which they contain. 5. Gas particlesno attraction to each other ...
Chapter 4 - The First Law of Thermodynamics and Energy Transport
... a while, so he asked him to add up all the numbers from 1 to 100. That is, find X = 1 + 2 + 3 + … + 100. To the teacher’s surprise, Gauss returned a few minutes later and said that the sum was 5050. Apparently Gauss noticed that the sum is the same regardless of whether the terms are added forward ( ...
... a while, so he asked him to add up all the numbers from 1 to 100. That is, find X = 1 + 2 + 3 + … + 100. To the teacher’s surprise, Gauss returned a few minutes later and said that the sum was 5050. Apparently Gauss noticed that the sum is the same regardless of whether the terms are added forward ( ...
Open questions (66 points total
... (NOTE There are 2 NMR spectra with this problem. Below the 1H spectrum, the integrals (= areas) of the signals are given as numbers ratios). From the IR spectrum of an unknown substance X with M = 102, we know X to be an ester. 6p 1 Calculate the molecular formula of substance X. Give all possible ...
... (NOTE There are 2 NMR spectra with this problem. Below the 1H spectrum, the integrals (= areas) of the signals are given as numbers ratios). From the IR spectrum of an unknown substance X with M = 102, we know X to be an ester. 6p 1 Calculate the molecular formula of substance X. Give all possible ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.