Ch 17--Thermochemistry(first class)
... 17.2: Enthalpy (measuring heat flow) Two types of calorimeters: a) Constant-Pressure calorimeter (eg. foam cups) • As most reactions occur at constant pressure we can say that: A change in enthalpy (ΔH) = heat supplied (q) • So, a release of heat (exothermic) corresponds to a decrease in enthalpy ( ...
... 17.2: Enthalpy (measuring heat flow) Two types of calorimeters: a) Constant-Pressure calorimeter (eg. foam cups) • As most reactions occur at constant pressure we can say that: A change in enthalpy (ΔH) = heat supplied (q) • So, a release of heat (exothermic) corresponds to a decrease in enthalpy ( ...
Salt Solutions Ionic Bonding
... NaCl is added to water. Like all equilibria, an equilibrium constant is equal to the ratio of the concentrations of products to the concentrations of reactants. The concentration of any solid is defined as 1. The concentration of pure water is a constant, 55.556 M. By multiplying the equilibrium con ...
... NaCl is added to water. Like all equilibria, an equilibrium constant is equal to the ratio of the concentrations of products to the concentrations of reactants. The concentration of any solid is defined as 1. The concentration of pure water is a constant, 55.556 M. By multiplying the equilibrium con ...
Introduction to Computational Chemistry
... course in computational chemistry. This material is elementary and does not require special skills or prior knowledge. It should be suitable for students after their second year in the chemistry curriculum. ...
... course in computational chemistry. This material is elementary and does not require special skills or prior knowledge. It should be suitable for students after their second year in the chemistry curriculum. ...
On the determination of the vapor–liquid envelope for polarizable
... case [3,4], most probably, due to inappropriate parameterization of current polarizable models. The goal of developing a polarizable model for water which yields an accurate phase envelope remains elusive and will continue to be until we can routinely and efficiently predict the phase envelope of po ...
... case [3,4], most probably, due to inappropriate parameterization of current polarizable models. The goal of developing a polarizable model for water which yields an accurate phase envelope remains elusive and will continue to be until we can routinely and efficiently predict the phase envelope of po ...
Department of Chemistry
... Statistical Thermodynamics 3.0; 3 cr. General statistical mechanics of independent particles; partition functions for atoms and molecules, and simple chemical equilibria; heat capacities of solids, configuration of polymers, ensembles, theory of imperfect gases and of mixtures, lattice statistics, ...
... Statistical Thermodynamics 3.0; 3 cr. General statistical mechanics of independent particles; partition functions for atoms and molecules, and simple chemical equilibria; heat capacities of solids, configuration of polymers, ensembles, theory of imperfect gases and of mixtures, lattice statistics, ...
Thermodynamics: Entropy, Free Energy and the Direction of
... (e) Seawater in midwinter at 20C or in midsummer at 230C (f) 1mol of CF4(g) or 1mol of CCl4(g) PLAN: In general less ordered systems have higher entropy than ordered systems and entropy increases with an increase in temperature. SOLUTION: (a) 1mol of SO3(g) - more atoms ...
... (e) Seawater in midwinter at 20C or in midsummer at 230C (f) 1mol of CF4(g) or 1mol of CCl4(g) PLAN: In general less ordered systems have higher entropy than ordered systems and entropy increases with an increase in temperature. SOLUTION: (a) 1mol of SO3(g) - more atoms ...
Pacing Guide, Revised Aug 17, 2010
... ICP.5.6 Identify key indicators of a chemical change and classify simple types of chemical reactions. Differentiate between covalent, ionic, hydrogen and Van der Waals bonding, and write formulas for and name compounds of each type. G) ICP.5.7 Explain that in exothermic chemical reactions chemical e ...
... ICP.5.6 Identify key indicators of a chemical change and classify simple types of chemical reactions. Differentiate between covalent, ionic, hydrogen and Van der Waals bonding, and write formulas for and name compounds of each type. G) ICP.5.7 Explain that in exothermic chemical reactions chemical e ...
Unit 4 - Calculations and Chemical Reactions
... Consider the reaction in which magnesium oxide reacts with carbon dioxide to form magnesium carbonate. We can represent the above “word description” by a “chemical equation”. Chemical equation: MgO + CO2 → MgCO3 Reactants Product We often indicate the physical state of reactants and products using t ...
... Consider the reaction in which magnesium oxide reacts with carbon dioxide to form magnesium carbonate. We can represent the above “word description” by a “chemical equation”. Chemical equation: MgO + CO2 → MgCO3 Reactants Product We often indicate the physical state of reactants and products using t ...
H - Workforce3One
... This product was funded by a grant awarded under the President’s High Growth Job Training Initiative, as implemented by the U.S. Department of Labor’s Employment & Training Administration. The information contained in this product was created by a grantee organization and does not necessarily reflec ...
... This product was funded by a grant awarded under the President’s High Growth Job Training Initiative, as implemented by the U.S. Department of Labor’s Employment & Training Administration. The information contained in this product was created by a grantee organization and does not necessarily reflec ...
Unit 5 Notes
... titration, which produces a dark blue colour (this is caused by the reaction between starch and the remaining I2 in solution). After adding the starch (which acts as a more noticeable and therefore more precise indicator), the last of the S2O32- is added, causing the blue colour of the starch-I2 mix ...
... titration, which produces a dark blue colour (this is caused by the reaction between starch and the remaining I2 in solution). After adding the starch (which acts as a more noticeable and therefore more precise indicator), the last of the S2O32- is added, causing the blue colour of the starch-I2 mix ...
Review Packet - Newton.k12.ma.us
... 5. - The molecular weight is the sum of the atomic weights of the atoms in a molecule of a compound. - The formula weight is the sum of the atomic weights of the atoms in a formula unit. - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quanti ...
... 5. - The molecular weight is the sum of the atomic weights of the atoms in a molecule of a compound. - The formula weight is the sum of the atomic weights of the atoms in a formula unit. - The molecular mass is the mass of one mole of any substance. 6. The advantage of using moles is that the quanti ...
The Ensembles
... = k log W (U, V, N ) Thus, as expected, the appropriate thermodynamic function is S(U, V, N ) which is a maximum at equilibrium for an isolated system. This also demonstrates that Boltzmann’s relation (eq. 6.4) applies to an isolated system. An important example of an isolated system that we have al ...
... = k log W (U, V, N ) Thus, as expected, the appropriate thermodynamic function is S(U, V, N ) which is a maximum at equilibrium for an isolated system. This also demonstrates that Boltzmann’s relation (eq. 6.4) applies to an isolated system. An important example of an isolated system that we have al ...
Thermochemistry ppt
... The heat change caused by dissolution of one mole of substance in the molar heat of solution (Hsoln). Sodium hydroxide is a good example of an exothermic molar heat of solution. When 1 mol of sodium hydroxide (NaOH)(s) is dissolved in water, the solution can become so hot that it steams. The heat i ...
... The heat change caused by dissolution of one mole of substance in the molar heat of solution (Hsoln). Sodium hydroxide is a good example of an exothermic molar heat of solution. When 1 mol of sodium hydroxide (NaOH)(s) is dissolved in water, the solution can become so hot that it steams. The heat i ...
model paper-1 - WordPress.com
... Using the gas equation, PV = nRT V=nRT/P =mRT/MP = 5x 0.082x323/26x0.9737 = 5.23 L 3M 14. a) The amount of heat absorbed or evolved when stoichiometric amounts of the reactants as represented by the balanced chemical equation have completely reacted under constant pressure conditions is termed as th ...
... Using the gas equation, PV = nRT V=nRT/P =mRT/MP = 5x 0.082x323/26x0.9737 = 5.23 L 3M 14. a) The amount of heat absorbed or evolved when stoichiometric amounts of the reactants as represented by the balanced chemical equation have completely reacted under constant pressure conditions is termed as th ...
Practice Final Exam, Chemistry 2220, Organic Chem II 1. Rank the
... 6. Which one of the following compounds is NOT a product of reaction between 1,3butadiene and HBr? A. (S)-3-bromo-1-butene B. (R)-3-bromo-1-butene C. (Z)-2-bromo-2-butene D. (E)-1-bromo-2-butene 7. Choose the reagents necessary to carry out the following conversion. O ...
... 6. Which one of the following compounds is NOT a product of reaction between 1,3butadiene and HBr? A. (S)-3-bromo-1-butene B. (R)-3-bromo-1-butene C. (Z)-2-bromo-2-butene D. (E)-1-bromo-2-butene 7. Choose the reagents necessary to carry out the following conversion. O ...
No Slide Title
... • The standard rate constant k0: It is the measure of the kinetic facility of a redox couple. A system with a large k0 will achieve equilibrium on a short time scale, but a system with small k0 will be sluggish • Values of k0 reported in the literature for electrochemical reactions vary from about 1 ...
... • The standard rate constant k0: It is the measure of the kinetic facility of a redox couple. A system with a large k0 will achieve equilibrium on a short time scale, but a system with small k0 will be sluggish • Values of k0 reported in the literature for electrochemical reactions vary from about 1 ...
Chemistry Pacing Guide - Michigan City Area Schools
... 2. Compounds are chemical combinations of elements. 3. Mixtures are physical combinations of substances that can be homogeneous or heterogeneous and can be separated by physical means. 4. Physical changes in matter do not change the identity of the matter. 5. Chemical changes in matter involve the f ...
... 2. Compounds are chemical combinations of elements. 3. Mixtures are physical combinations of substances that can be homogeneous or heterogeneous and can be separated by physical means. 4. Physical changes in matter do not change the identity of the matter. 5. Chemical changes in matter involve the f ...
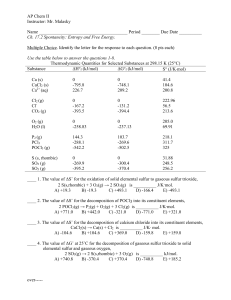
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.