The Equilibrium Constant
... • A catalyst speeds up the RATE of a reaction by lowering the ACTIVATION ENERGY. • Activation energy is lowered the same amount for the forward and reverse reactions. • Therefore, a catalyst does not affect the position of equilibrium, Kc. ...
... • A catalyst speeds up the RATE of a reaction by lowering the ACTIVATION ENERGY. • Activation energy is lowered the same amount for the forward and reverse reactions. • Therefore, a catalyst does not affect the position of equilibrium, Kc. ...
Exam #2
... Given that Hf for MgCl2 is -641.6 kJ/mol and Hf for KCl is -435.9 kJ/mol, calculate Hrxn for the following equation: ...
... Given that Hf for MgCl2 is -641.6 kJ/mol and Hf for KCl is -435.9 kJ/mol, calculate Hrxn for the following equation: ...
First 9 weeks Study Guide 8th Grade
... Nobel Gases have a full shell so they are stable and will not react with other element’s atoms. The have 8 valence electrons. ...
... Nobel Gases have a full shell so they are stable and will not react with other element’s atoms. The have 8 valence electrons. ...
Chemistry2 Midterm Review 2012 – Tuesday
... a. What is the molar mass of allicin? b. How many moles of allicin are present in 5.00 mg of this substance? c. How many molecules of allicin are in 5.00 mg of this substance? d. How many S atoms are present in 5.00 mg of allicin? 32. a. How many moles of ammonium ions are in 0.557 g of ammonium car ...
... a. What is the molar mass of allicin? b. How many moles of allicin are present in 5.00 mg of this substance? c. How many molecules of allicin are in 5.00 mg of this substance? d. How many S atoms are present in 5.00 mg of allicin? 32. a. How many moles of ammonium ions are in 0.557 g of ammonium car ...
Thermodynamics of Skeletal Muscle During Cardiocirculatory Assist
... world [14, 15]. With the increased use of recruited muscular power for biomechanical assistance [21] the challenge biology faces for the XXI century is to apply the discipline of thermodynamics to the performance of complex living systems. Bertalanffy [1], Lehninger [18], and Nicolis and Prigogine [ ...
... world [14, 15]. With the increased use of recruited muscular power for biomechanical assistance [21] the challenge biology faces for the XXI century is to apply the discipline of thermodynamics to the performance of complex living systems. Bertalanffy [1], Lehninger [18], and Nicolis and Prigogine [ ...
review CH5 chem121pikul
... Step [1] Write the equation with the correct formulas. • The subscripts in a formula can never be changed to balance an equation, because changing a subscript changes the identity of a compound. ...
... Step [1] Write the equation with the correct formulas. • The subscripts in a formula can never be changed to balance an equation, because changing a subscript changes the identity of a compound. ...
Specification
... The term, ‘number of moles’ is to be avoided. The term, ‘amount of substance in moles’ is preferred. In the same manner, the size of an object can be described in terms of its ‘length in metres’, rather than its ‘number of metres’. Graph Axes and Table Headings Labelled as: quantity / unit, e.g. c / ...
... The term, ‘number of moles’ is to be avoided. The term, ‘amount of substance in moles’ is preferred. In the same manner, the size of an object can be described in terms of its ‘length in metres’, rather than its ‘number of metres’. Graph Axes and Table Headings Labelled as: quantity / unit, e.g. c / ...
Chapter 5
... Energy can be transferred between a system and its surrounding in the form of heat (q) and/or work (w) Work = Force Distance ( w = f d ) Energy (work) is transferred from surrounding to an object (system) when the object is moved by external force. ...
... Energy can be transferred between a system and its surrounding in the form of heat (q) and/or work (w) Work = Force Distance ( w = f d ) Energy (work) is transferred from surrounding to an object (system) when the object is moved by external force. ...
Lab 3. Chemical Reactions
... When elements or compounds chemically react to form products, no material (matter) is lost or gained. All of the atoms used as reactants are converted into products. Every atom of every element must be accounted for since they are not destroyed or created, just rearranged and recombined into new thi ...
... When elements or compounds chemically react to form products, no material (matter) is lost or gained. All of the atoms used as reactants are converted into products. Every atom of every element must be accounted for since they are not destroyed or created, just rearranged and recombined into new thi ...
PDF(343KB)
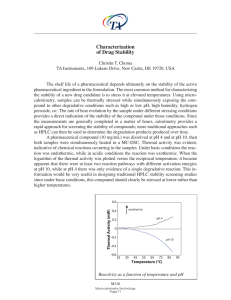
... rapid approach for screening the stability of compounds; more traditional approaches such as HPLC can then be used to determine the degradation products produced over time. A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-D ...
... rapid approach for screening the stability of compounds; more traditional approaches such as HPLC can then be used to determine the degradation products produced over time. A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-D ...
Energy - iheartchem
... • The potential energy lost from the burned match came from the stored energy in the bonds of the reactants • In any exothermic reaction some of the potential energy stored in the chemical bonds is converted to thermal energywhich is random kinetic energy- as heat ...
... • The potential energy lost from the burned match came from the stored energy in the bonds of the reactants • In any exothermic reaction some of the potential energy stored in the chemical bonds is converted to thermal energywhich is random kinetic energy- as heat ...
MIDDLE COLLEGE HIGH SCHOOL
... Base your answers to questions 79 and 80 on the information and equation below. Human blood contains dissolved carbonic acid, H2CO3, in equilibrium with carbon dioxide and water. The equilibrium system is shown below. H2CO3(aq) CO2(aq) + H2O(ℓ) 61. Explain, using LeChatelier’s principle, why decreas ...
... Base your answers to questions 79 and 80 on the information and equation below. Human blood contains dissolved carbonic acid, H2CO3, in equilibrium with carbon dioxide and water. The equilibrium system is shown below. H2CO3(aq) CO2(aq) + H2O(ℓ) 61. Explain, using LeChatelier’s principle, why decreas ...
documentstyle[12pt]{article}
... Chapter 1 Lecture notes for Thermodynamics: An Engineering Approach, 3rd Ed by Cengel and Boles ...
... Chapter 1 Lecture notes for Thermodynamics: An Engineering Approach, 3rd Ed by Cengel and Boles ...
B.S. in Chemical Engineering – Sample Curriculum
... *1 A minimum of 126 units is required for the BS in ChE degree; this chart represents one example. Check all pre-reqs and speak with adviser(s). *2 Intro to Engineering Tools: MATLAB and Simulink is recommended, but not required. *3 Humanities & Social Science electives: 18 H/SS units required; all ...
... *1 A minimum of 126 units is required for the BS in ChE degree; this chart represents one example. Check all pre-reqs and speak with adviser(s). *2 Intro to Engineering Tools: MATLAB and Simulink is recommended, but not required. *3 Humanities & Social Science electives: 18 H/SS units required; all ...
Lesson 9 Review Teacher`s Copy
... Chemistry[2015-2016 Redox Practice Test[4/27/2016]]- New York ...
... Chemistry[2015-2016 Redox Practice Test[4/27/2016]]- New York ...
Master Equation Solver for Multi-Energy well Reactions
... • Divide energy of species into grains. • Generate a set of coupled differential equations involving source terms, reaction forward/backward and energy transfer. C** ...
... • Divide energy of species into grains. • Generate a set of coupled differential equations involving source terms, reaction forward/backward and energy transfer. C** ...
Chemistry Syllabus
... 3a. Calculate the number of protons, neutrons, and electrons in individual isotopes using atomic numbers and mass numbers, and write electron configurations of elements and ions following the Aufbau principle. (DOK 1) 3b. Analyze patterns and trends in the organization of elements in the periodic ta ...
... 3a. Calculate the number of protons, neutrons, and electrons in individual isotopes using atomic numbers and mass numbers, and write electron configurations of elements and ions following the Aufbau principle. (DOK 1) 3b. Analyze patterns and trends in the organization of elements in the periodic ta ...
Chemistry Syllabus - Madison County Schools
... 4b. Use the ideal gas laws to explain the relationships between volume, temperature, pressure, and quantity in moles. (DOK 2) Difference between ideal and real gas Assumptions made about an ideal gas Conditions that favor an ideal gas 4c. Use the gas laws of Boyle, Charles, Gay-Lussac and Dalt ...
... 4b. Use the ideal gas laws to explain the relationships between volume, temperature, pressure, and quantity in moles. (DOK 2) Difference between ideal and real gas Assumptions made about an ideal gas Conditions that favor an ideal gas 4c. Use the gas laws of Boyle, Charles, Gay-Lussac and Dalt ...
balancing chemical equations worksheet
... Q2. A balanced chemical equation can be written in four steps. 1. Write the reaction in words, 2. Change the chemical names into their correct symbols and formulae. 3. Include the physical states and 4. finally balance. The following questions relate to these four steps. a. What symbols should we us ...
... Q2. A balanced chemical equation can be written in four steps. 1. Write the reaction in words, 2. Change the chemical names into their correct symbols and formulae. 3. Include the physical states and 4. finally balance. The following questions relate to these four steps. a. What symbols should we us ...
Chemical and Molecular Formulas PPT
... hormones in your body, and others are ionic, such as the salts in body fluids ...
... hormones in your body, and others are ionic, such as the salts in body fluids ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.












![documentstyle[12pt]{article}](http://s1.studyres.com/store/data/010234315_1-392ad57a1bf5b2aaeca94206588a5307-300x300.png)