Chapter 6 ()
... additional information to deal with the state variables density and pressure and that we were one equation short of matching unknowns and equations. In both meteorology and oceanography the variation of density and hence buoyancy is critical in many phenomenon such cyclogenesis and the thermohaline ...
... additional information to deal with the state variables density and pressure and that we were one equation short of matching unknowns and equations. In both meteorology and oceanography the variation of density and hence buoyancy is critical in many phenomenon such cyclogenesis and the thermohaline ...
Chapter 6
... possible to nevertheless accept this unnecessary richness but the momentum, thermodynamic and mass conservation equations are each nonlinear because of the advective derivative so a frontal attack on the full equations, even with the most powerful modern computers is a hopeless approach. This is bot ...
... possible to nevertheless accept this unnecessary richness but the momentum, thermodynamic and mass conservation equations are each nonlinear because of the advective derivative so a frontal attack on the full equations, even with the most powerful modern computers is a hopeless approach. This is bot ...
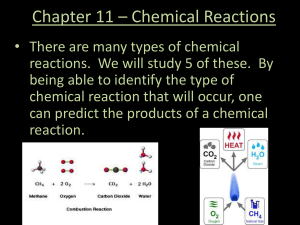
Chapter 11 * Chemical Reactions
... Chemical Reactions • Chemical equations • Balancing Chemical Equations • According to the Law of Conservation of Mass, the number of atoms on the reactant side of the equation must equal the number of atoms on the product side. • We can adjust the number of atoms on each side of the equation by pla ...
... Chemical Reactions • Chemical equations • Balancing Chemical Equations • According to the Law of Conservation of Mass, the number of atoms on the reactant side of the equation must equal the number of atoms on the product side. • We can adjust the number of atoms on each side of the equation by pla ...
Academic Chemistry Final Exam Review
... a. EXAMPLE: The unit abbreviation “m” stands for ____meter___ and is a unit of ___length___. b. The unit abbreviation “g” stands for ________________ and is a unit of _______________. c. The unit abbreviation “mL” stands for _____________________ and is a unit of ________________. d. The unit abbrev ...
... a. EXAMPLE: The unit abbreviation “m” stands for ____meter___ and is a unit of ___length___. b. The unit abbreviation “g” stands for ________________ and is a unit of _______________. c. The unit abbreviation “mL” stands for _____________________ and is a unit of ________________. d. The unit abbrev ...
Ch 3 Matter & Change
... Each one has a unique name and symbol. In the symbol the first letter is always capitalized and the remaining letter(s) are lowercase. There are 91 naturally occurring elements Who was given credit for organizing them into a table? Dmitri Mendeleev ...
... Each one has a unique name and symbol. In the symbol the first letter is always capitalized and the remaining letter(s) are lowercase. There are 91 naturally occurring elements Who was given credit for organizing them into a table? Dmitri Mendeleev ...
Equilibrium Constant
... so substitution may be necessary! Must be found experimentally or by means of equilibrium concentrations from thermodynamic data. Also varies with temperature, and constant at a given temperature (just like kinetic rate constants) Independent of initial concentration ...
... so substitution may be necessary! Must be found experimentally or by means of equilibrium concentrations from thermodynamic data. Also varies with temperature, and constant at a given temperature (just like kinetic rate constants) Independent of initial concentration ...
Syllabus
... INTRODUCTION AND LEARNING GOALS - Whether we like it or not, we live in a dynamic chemical universe. Chemical properties and reactions influence our every action (and reaction). We rely upon chemical properties and reactions to both sustain and cultivate our lives. This course is intended to provide ...
... INTRODUCTION AND LEARNING GOALS - Whether we like it or not, we live in a dynamic chemical universe. Chemical properties and reactions influence our every action (and reaction). We rely upon chemical properties and reactions to both sustain and cultivate our lives. This course is intended to provide ...
Syracuse University
... INTRODUCTION AND LEARNING GOALS - Whether we like it or not, we live in a dynamic chemical universe. Chemical properties and reactions influence our every action (and reaction). We rely upon chemical properties and reactions to both sustain and cultivate our lives. This course is intended to provide ...
... INTRODUCTION AND LEARNING GOALS - Whether we like it or not, we live in a dynamic chemical universe. Chemical properties and reactions influence our every action (and reaction). We rely upon chemical properties and reactions to both sustain and cultivate our lives. This course is intended to provide ...
Unit 6 Naming Binary Compounds
... A combustion reaction is a chemical change in which an element or a compound reacts with oxygen, often producing energy in the form of heat and light. ...
... A combustion reaction is a chemical change in which an element or a compound reacts with oxygen, often producing energy in the form of heat and light. ...
Welcome back to Chemistry!
... One of the most important skills of a chemist! It allows us to detect chemical and physical changes. The heart of chemistry. ...
... One of the most important skills of a chemist! It allows us to detect chemical and physical changes. The heart of chemistry. ...
Lighting the Way
... • Infrared lamps produce heat for personal and industrial use. Now that feels better than saving money! ...
... • Infrared lamps produce heat for personal and industrial use. Now that feels better than saving money! ...
Chemical Reactions and Stoichiometry
... Indicators of a Chemical Reaction – evidence of a chemical reaction a. Evolution of heat and light (simultaneously) b. Production of a gas (bubbles, odor change) c. Formation of a precipitate (solid, cloudy) d. Color change (not introduced by an outside source such as dye or ink) Characteristics of ...
... Indicators of a Chemical Reaction – evidence of a chemical reaction a. Evolution of heat and light (simultaneously) b. Production of a gas (bubbles, odor change) c. Formation of a precipitate (solid, cloudy) d. Color change (not introduced by an outside source such as dye or ink) Characteristics of ...
Phase Changes and latent heat
... The ideal gas law is a special form of an equation of state state, i.e., an equation relating the variables that characterize a gas (pressure, volume, temperature, density, ….). The ideal gas law is applicable to low-density gases. ...
... The ideal gas law is a special form of an equation of state state, i.e., an equation relating the variables that characterize a gas (pressure, volume, temperature, density, ….). The ideal gas law is applicable to low-density gases. ...
Fall Final Review Honors
... STRATEGY: Start by reading through your notes to refresh your memory on these topics. Then, use this review sheet as a starting point to identify the areas on which you need to spend more study time. For those areas, go back to homework assignments, quizzes, and reviews to practice more problems. I ...
... STRATEGY: Start by reading through your notes to refresh your memory on these topics. Then, use this review sheet as a starting point to identify the areas on which you need to spend more study time. For those areas, go back to homework assignments, quizzes, and reviews to practice more problems. I ...
1. What are micelles? Give two examples of micellar systems. Sol. A
... energetically preferred orientation has the magnetic moment aligned parallel with the applied field (spin +1/2) and is often given the notation , whereas the higher energy anti-parallel orientation (spin -1/2) is referred to as . The rotational axis of the spinning nucleus cannot be orientated exact ...
... energetically preferred orientation has the magnetic moment aligned parallel with the applied field (spin +1/2) and is often given the notation , whereas the higher energy anti-parallel orientation (spin -1/2) is referred to as . The rotational axis of the spinning nucleus cannot be orientated exact ...
ExamView - test.practice.questions.tst
... 1. 2.1 – WWBAT tell apart different states of matter on an atomic basis (3.1) The random molecular motion of a substance is greatest when the substance is a. condensed. c. frozen. b. a liquid. d. a gas. 2. 2.1 – WWBAT tell apart different states of matter on an atomic basis Under the same conditions ...
... 1. 2.1 – WWBAT tell apart different states of matter on an atomic basis (3.1) The random molecular motion of a substance is greatest when the substance is a. condensed. c. frozen. b. a liquid. d. a gas. 2. 2.1 – WWBAT tell apart different states of matter on an atomic basis Under the same conditions ...
NOTES on THERMODYNAMICS - University of Utah Physics
... ⋆ As a phenomenological description, it is based on a number of empirical observations which are summarized by the laws of thermodynamics. A coherent logical and mathe matical structure is then constructed on the basis of these observations, which leads to a variety of useful concepts, and to testa ...
... ⋆ As a phenomenological description, it is based on a number of empirical observations which are summarized by the laws of thermodynamics. A coherent logical and mathe matical structure is then constructed on the basis of these observations, which leads to a variety of useful concepts, and to testa ...
Document
... First Law for a Control Volume For a reacting flow, a control volume is generally used, and the equivalent form of the above equation at steady state is ...
... First Law for a Control Volume For a reacting flow, a control volume is generally used, and the equivalent form of the above equation at steady state is ...
Ideal gas - Let`s Enjoy Chemical Engineering World
... Consider a system consisting of 10 g of liquid water contained in an open beaker under constant pressure of 1 atm. Initially the water is at 25 °C the initial state : p = 1 atm, t = 25 °C The system is contacted with 100 g of water at a high temperature, 90 °C. The system is kept in contact with thi ...
... Consider a system consisting of 10 g of liquid water contained in an open beaker under constant pressure of 1 atm. Initially the water is at 25 °C the initial state : p = 1 atm, t = 25 °C The system is contacted with 100 g of water at a high temperature, 90 °C. The system is kept in contact with thi ...
Chemistry Final Exam Review 2006-2007
... 23. Determine the pH of a 2.0 x 10-2 M Sr(OH)2? 24. The pH of a solution is measured and determined to be 7.52? What is the hydrogen ion concentration? Is the solution acidic or basic? Objective 6.5A, B & C 1. What do the coefficients mean in a chemical equation? 2. Calculate the mole ratio between ...
... 23. Determine the pH of a 2.0 x 10-2 M Sr(OH)2? 24. The pH of a solution is measured and determined to be 7.52? What is the hydrogen ion concentration? Is the solution acidic or basic? Objective 6.5A, B & C 1. What do the coefficients mean in a chemical equation? 2. Calculate the mole ratio between ...
Inorganic Chemistry
... quantities, A and G as criteria for thermodynamic equilibrium and spontaneity, their advantage over entropy change. Variation of G and A with P, V and T. Gibbs-Helmoltz equation, Clapeyron equation, Clausius-Clapeyron equation, reaction isotherm and reaction isochore. 2. Chemical Equilibrium 06 hrs ...
... quantities, A and G as criteria for thermodynamic equilibrium and spontaneity, their advantage over entropy change. Variation of G and A with P, V and T. Gibbs-Helmoltz equation, Clapeyron equation, Clausius-Clapeyron equation, reaction isotherm and reaction isochore. 2. Chemical Equilibrium 06 hrs ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.