...detail
... Idea of distribution functions. Properties of Gamma functions; transformation properties for Cartesian to polar coordinates. Maxwell’s speed and energy distributions (derivations for 1, 2 and 3 dimensions); distribution curves; different types of speeds and their significance. Frequency of collision ...
... Idea of distribution functions. Properties of Gamma functions; transformation properties for Cartesian to polar coordinates. Maxwell’s speed and energy distributions (derivations for 1, 2 and 3 dimensions); distribution curves; different types of speeds and their significance. Frequency of collision ...
(a) T
... For closed case, the volume of the system changes, the PV work is involved; for open case, the enthalpy which contain the flow work is involved. Somehow, PV work is related to flow work. PV work (closed) = Flow work (open) ...
... For closed case, the volume of the system changes, the PV work is involved; for open case, the enthalpy which contain the flow work is involved. Somehow, PV work is related to flow work. PV work (closed) = Flow work (open) ...
Beginning Research on the Quantification of Spatial Order
... Whereas the First Law of thermodynamics states that total energy is conserved, albeit in altered form, the Second Law pertains to the way in which energy might change form, as regards the potential to do useful work, in the manner that a heat engine does useful mechanical work. In 1867 Clausius expr ...
... Whereas the First Law of thermodynamics states that total energy is conserved, albeit in altered form, the Second Law pertains to the way in which energy might change form, as regards the potential to do useful work, in the manner that a heat engine does useful mechanical work. In 1867 Clausius expr ...
Review Packet Honors Chemistry Kovacs
... _____1. Which of the following describes nuclear fusion? a. 2 small atoms form one big atom d. A Molecule breaks a bond, forming 2 atoms b. 2 atoms form a chemical bond to form a molecule e. all of them c. One big atom breaks into 2 smaller atoms F. Just A,B, and C _____2. Which of the following is ...
... _____1. Which of the following describes nuclear fusion? a. 2 small atoms form one big atom d. A Molecule breaks a bond, forming 2 atoms b. 2 atoms form a chemical bond to form a molecule e. all of them c. One big atom breaks into 2 smaller atoms F. Just A,B, and C _____2. Which of the following is ...
Chapter 15. Chemical Equilibrium
... The equilibrium expression depends on stoichiometry. • It does not depend on the reaction mechanism. • The value of Kc varies with temperature. We generally omit the units of the equilibrium constant. ...
... The equilibrium expression depends on stoichiometry. • It does not depend on the reaction mechanism. • The value of Kc varies with temperature. We generally omit the units of the equilibrium constant. ...
E 0
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
The Second Law: Definition of Entropy
... powered by steam, but it turned out to be quite difficult to build one that was efficient enough to get anything done! In an engine engine, there is a cycle in which fuel is burned to heat gas inside the piston. The expansion of the piston leads to cooling and work. Compression readies the piston fo ...
... powered by steam, but it turned out to be quite difficult to build one that was efficient enough to get anything done! In an engine engine, there is a cycle in which fuel is burned to heat gas inside the piston. The expansion of the piston leads to cooling and work. Compression readies the piston fo ...
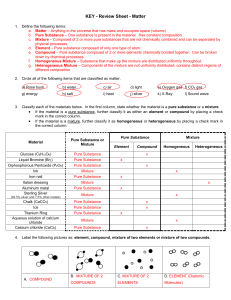
Matter Test Review Sheet
... e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by chemical processes. f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Heterogeneous Mixture – Components of the mixture are not uniformly d ...
... e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by chemical processes. f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Heterogeneous Mixture – Components of the mixture are not uniformly d ...
Unit 1 PowerPoint Complete Notes
... Determining the Physical States To determine if a product is a solid or not, use a table of solubility. The following are hints to help determine the physical state. (s) – most metals, precipitates (l) – mercury, bromine, water (g) – noble gases, diatomic molecules (except bromine), ammonia (aq) – ...
... Determining the Physical States To determine if a product is a solid or not, use a table of solubility. The following are hints to help determine the physical state. (s) – most metals, precipitates (l) – mercury, bromine, water (g) – noble gases, diatomic molecules (except bromine), ammonia (aq) – ...
Heat of Sublimation - Chemwiki
... kJ/kg, then calculate the heat of sublimation for 1.00 kg of H2O(s) with the initial temperature, 273K (Hint: 273K is the solid-liquid phase change temperature and 373K is the liquid-gas phase change temperature). Using the information given in question one, calculate the heat of sublimation for 1. ...
... kJ/kg, then calculate the heat of sublimation for 1.00 kg of H2O(s) with the initial temperature, 273K (Hint: 273K is the solid-liquid phase change temperature and 373K is the liquid-gas phase change temperature). Using the information given in question one, calculate the heat of sublimation for 1. ...
Chapter 4
... - acetic acid can act as a differentiating solvent in which various acids dissociate to different degrees and thus have different strengths, while water acts as a leveling solvent for strong acids because the strong acid will dissociate completely and have no differences in strength. F. Chemical Equ ...
... - acetic acid can act as a differentiating solvent in which various acids dissociate to different degrees and thus have different strengths, while water acts as a leveling solvent for strong acids because the strong acid will dissociate completely and have no differences in strength. F. Chemical Equ ...
... Nitrogen gas is compressed in a steady-state, steady-flow, adiabatic process from 0.1 MPa, 25oC. During the compression process the temperature becomes 125oC. If the mass flow rate is 0.2 kg/s, determine the work done on the nitrogen, in kW. Control volume: The compressor (see the compressor sketche ...
File - Science With BLT
... a. coefficients of the reactants equal the coefficients of the products. b. same number of each kind of atom appears in the reactants and in the products. c. products and reactants are the same chemicals. d. subscripts of the reactants equal the subscripts of the products. ...
... a. coefficients of the reactants equal the coefficients of the products. b. same number of each kind of atom appears in the reactants and in the products. c. products and reactants are the same chemicals. d. subscripts of the reactants equal the subscripts of the products. ...
THE FREE ENERGIES OF FORMATION OF AQUEOUS d
... energy between initial and final states (i.e. F1 - F,), and A(PV) the change in the pressure-volume product, P2V2 - PIV1. Further in any spontaneous process - AF is a positive quantity. Let us consider the hypothetical reaction 2c ...
... energy between initial and final states (i.e. F1 - F,), and A(PV) the change in the pressure-volume product, P2V2 - PIV1. Further in any spontaneous process - AF is a positive quantity. Let us consider the hypothetical reaction 2c ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.