
English Medium
... 2. Explain why dogs pant during hot summer days using the concept of evaporation? A. Dogs do not have sweat pores on their skin, but only at their feet. The sweat produced by evaporation would not have way to go out. Hence it pant during hot summer days to maintain its body temperature balance. 3. C ...
... 2. Explain why dogs pant during hot summer days using the concept of evaporation? A. Dogs do not have sweat pores on their skin, but only at their feet. The sweat produced by evaporation would not have way to go out. Hence it pant during hot summer days to maintain its body temperature balance. 3. C ...
AP Chemistry 2015-2016 Name: Chapter 5: Thermodynamics Date
... state the difference between system and surroundings. recognize the system and the surroundings in a chemical or physical system. calculate the change in internal energy based on changes in heat absorbed by the system and work done by the system. state that H is a more general (and useful) ...
... state the difference between system and surroundings. recognize the system and the surroundings in a chemical or physical system. calculate the change in internal energy based on changes in heat absorbed by the system and work done by the system. state that H is a more general (and useful) ...
spontaneous change: entropy and free energy
... from iron(III) oxide. We should not say that the process is impossible, but it is certainly nonspontaneous. In fact, this nonspontaneous reverse process is involved in the manufacture of iron from iron ore. We will consider specific quantitative criteria for spontaneous change later in the chapter, ...
... from iron(III) oxide. We should not say that the process is impossible, but it is certainly nonspontaneous. In fact, this nonspontaneous reverse process is involved in the manufacture of iron from iron ore. We will consider specific quantitative criteria for spontaneous change later in the chapter, ...
Chemistry 140
... 1. Make sure that the measured quantity has a decimal point. 2. Start at the left of the number and move right until you reach the first nonzero digit. 3. Count that digit and every digit to it’s right as significant. Zeros that end a number and lie either after or before the decimal point are signi ...
... 1. Make sure that the measured quantity has a decimal point. 2. Start at the left of the number and move right until you reach the first nonzero digit. 3. Count that digit and every digit to it’s right as significant. Zeros that end a number and lie either after or before the decimal point are signi ...
ch20powerpoint
... ∆G and work a system can do: ∆G < 0, for a spontaneous process., ∆G=-wmax, maximum useful work obtainable from the system. ∆G > 0, for a nonspontaneous process., minimum work that must be done to the system to make the process take place. In any real process work is performed irreversibly and so ...
... ∆G and work a system can do: ∆G < 0, for a spontaneous process., ∆G=-wmax, maximum useful work obtainable from the system. ∆G > 0, for a nonspontaneous process., minimum work that must be done to the system to make the process take place. In any real process work is performed irreversibly and so ...
CHAPTER I
... the state is in the compressed liquid region, and the compressed liquid tables are used to find the properties of the state. If the answer to the second question is yes, the state is in the saturation region, and either the saturation temperature table or the saturation pressure table is used to fin ...
... the state is in the compressed liquid region, and the compressed liquid tables are used to find the properties of the state. If the answer to the second question is yes, the state is in the saturation region, and either the saturation temperature table or the saturation pressure table is used to fin ...
Lecture 3 - Fluid Dynamics and Balance Equations
... • Let us consider a general quantity per unit volume f(x, t). Its integral over the finite volume V, with the time-independent boundary A is given by ...
... • Let us consider a general quantity per unit volume f(x, t). Its integral over the finite volume V, with the time-independent boundary A is given by ...
Introduction to Thermochemistry and Specific Heat
... • The amount of heat required to convert one mole of the substance from one phase to another is its molar enthalpy of transition (DHfus, DHvap, DHsub). What is the sign for all three? +DH • The amount of heat given off for one mole of a substance during a phase transition while cooling is its molar ...
... • The amount of heat required to convert one mole of the substance from one phase to another is its molar enthalpy of transition (DHfus, DHvap, DHsub). What is the sign for all three? +DH • The amount of heat given off for one mole of a substance during a phase transition while cooling is its molar ...
Final Exam SG Part 1 (Unit 5).
... c. How many moles are produced from the moles of the reactants? d. If you double the amount of white molecules (so now you have 8 pairs) but keep the same amount of black molecules, how many molecules can you produce? 4. Percent Yield a. ___Sb4O6 + ____C → ____Sb + ____CO Determine the percent yield ...
... c. How many moles are produced from the moles of the reactants? d. If you double the amount of white molecules (so now you have 8 pairs) but keep the same amount of black molecules, how many molecules can you produce? 4. Percent Yield a. ___Sb4O6 + ____C → ____Sb + ____CO Determine the percent yield ...
Ch. 15 Study Guide
... 30. Chemical equations tell us the net change, but they don’t tell us anything about how the reaction happens. 31. Most reactions occur in a series of small steps that leads to the final products. The series of steps is known as the mechanism of the reaction. ...
... 30. Chemical equations tell us the net change, but they don’t tell us anything about how the reaction happens. 31. Most reactions occur in a series of small steps that leads to the final products. The series of steps is known as the mechanism of the reaction. ...
4 Expressing and Measuring Chemical Change
... occurs remains constant. Priestley and Lavoisier were both radical thinkers for the time they lived in. Englishman Priestley’s support of the French Revolutionists led to his persecution, and he eventually had to flee from Europe altogether. He lived the last of his days in Pennsylvania in the Unite ...
... occurs remains constant. Priestley and Lavoisier were both radical thinkers for the time they lived in. Englishman Priestley’s support of the French Revolutionists led to his persecution, and he eventually had to flee from Europe altogether. He lived the last of his days in Pennsylvania in the Unite ...
[SESSION-2014-2015] SUBJECT - SCIENCE PATNA REGION
... A water insoluble substance ‗X‘ on reacting with dilute H2SO4 released a colourless and odourless gas accompanied by brisk effervescence. When the gas was passed through water, the solution obtained turned blue litmus red. On bubbling the gas through lime water, it initially became milky and milkyne ...
... A water insoluble substance ‗X‘ on reacting with dilute H2SO4 released a colourless and odourless gas accompanied by brisk effervescence. When the gas was passed through water, the solution obtained turned blue litmus red. On bubbling the gas through lime water, it initially became milky and milkyne ...
Enzyme Activity
... specific chemical reaction without itself being destroyed or changed in any way. • K m: (Michaelis constant) The substrate concentration at which an enzyme catalysed reaction proceeds at half the maximum ...
... specific chemical reaction without itself being destroyed or changed in any way. • K m: (Michaelis constant) The substrate concentration at which an enzyme catalysed reaction proceeds at half the maximum ...
Number of Electron Pairs Allowed Sigmatropic Rearrangement
... 1. The number of MO’s equals the number of AO’s combining to form them. 2. The energies of the MO’s are symmetrically placed about the energy of an isolated p orbital (arbitrarily taken as zero energy). 3. The energy of an MO increases as the number of nodes increases. 4. Nodes are symmetrically pla ...
... 1. The number of MO’s equals the number of AO’s combining to form them. 2. The energies of the MO’s are symmetrically placed about the energy of an isolated p orbital (arbitrarily taken as zero energy). 3. The energy of an MO increases as the number of nodes increases. 4. Nodes are symmetrically pla ...
Document
... calorimeter, the temperature of the resultant solution increases from 21.0 0C to 27.50C. Calculate the enthalpy change for the reaction in kJ/mol HCl, assuming that the calorimeter loses only a negligible quantity of heat, that the total volume of the solution is 100.0 mL, that its density is 1.00 g ...
... calorimeter, the temperature of the resultant solution increases from 21.0 0C to 27.50C. Calculate the enthalpy change for the reaction in kJ/mol HCl, assuming that the calorimeter loses only a negligible quantity of heat, that the total volume of the solution is 100.0 mL, that its density is 1.00 g ...
6.02 × 1023 molecules = 1 mole
... One of the most well-known numbers in the study of chemistry is number of units in a mole. The number of units in a mole is called Avogadro’s number (named after the Italian physicist). The mole is defined as the number of atoms in 12.0 grams of 12C. As you can tell from the equality below, the mole ...
... One of the most well-known numbers in the study of chemistry is number of units in a mole. The number of units in a mole is called Avogadro’s number (named after the Italian physicist). The mole is defined as the number of atoms in 12.0 grams of 12C. As you can tell from the equality below, the mole ...
Chemical Equilibrium - Shailendra Kumar Chemistry
... For an equilibrium change involving gaseous phase, the forward reaction is first order while the reverse reaction is second order. The unit of Kp for the forward equilibrium is : ...
... For an equilibrium change involving gaseous phase, the forward reaction is first order while the reverse reaction is second order. The unit of Kp for the forward equilibrium is : ...
Chemical thermodynamics

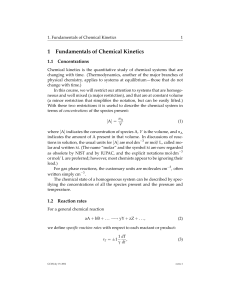
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.














![[SESSION-2014-2015] SUBJECT - SCIENCE PATNA REGION](http://s1.studyres.com/store/data/008930072_1-5a35e1ae8e3204ea88999f1418a93013-300x300.png)






