Instructor`s Resource Manual
... with its appropriate division into parts, sections, and subsections, allows for flexible rearrangement to meet individual syllabus configurations. To smooth the process of teaching with the text, we have worked diligently in several areas. Each technical term is clearly defined at first mention, and ...
... with its appropriate division into parts, sections, and subsections, allows for flexible rearrangement to meet individual syllabus configurations. To smooth the process of teaching with the text, we have worked diligently in several areas. Each technical term is clearly defined at first mention, and ...
FREE Sample Here
... B) molecules in the gas phase have more potential energy than in solids. C) molecules in the gas phase have more kinetic energy than in solids. D) gaseous molecules have less mass. E) molecules in the gas phase have more space between them than in solids. Ans: E Difficulty: M ...
... B) molecules in the gas phase have more potential energy than in solids. C) molecules in the gas phase have more kinetic energy than in solids. D) gaseous molecules have less mass. E) molecules in the gas phase have more space between them than in solids. Ans: E Difficulty: M ...
Kinetic modelling of the Maillard reaction between proteins and sugars
... monosaccharide-casein systems, which were heated at 120°C and neutral pH, were identified and quantified, and thereaction pathways were established. Themain reaction routes were (i) sugar isomerisation, (ii) degradation of the sugar into carboxylic acids and (iii) the Maillard reaction itself, in wh ...
... monosaccharide-casein systems, which were heated at 120°C and neutral pH, were identified and quantified, and thereaction pathways were established. Themain reaction routes were (i) sugar isomerisation, (ii) degradation of the sugar into carboxylic acids and (iii) the Maillard reaction itself, in wh ...
Chemical Quantities
... antacid. The company claims that its product neutralizes 10 times as much stomach acid per tablet as its nearest competitor. How would you test the validity of this claim? Or suppose that after graduation you go to work for a chemical company that makes methanol (methyl alcohol), a substance used as ...
... antacid. The company claims that its product neutralizes 10 times as much stomach acid per tablet as its nearest competitor. How would you test the validity of this claim? Or suppose that after graduation you go to work for a chemical company that makes methanol (methyl alcohol), a substance used as ...
CYPRUS
... The Chemistry Undergraduate Program includes introductory Chemistry courses (Organic, Inorganic, Analytical and Physical Chemistry), Physics, Mathematics and Information Technology during the first four semesters. The last four semesters help the students to enrich their knowledge in advanced concep ...
... The Chemistry Undergraduate Program includes introductory Chemistry courses (Organic, Inorganic, Analytical and Physical Chemistry), Physics, Mathematics and Information Technology during the first four semesters. The last four semesters help the students to enrich their knowledge in advanced concep ...
Modern Chemistry
... 1. Determine whether each of the following is an example of observation and data, a theory, a hypothesis, a control, or a model. a. A research team records the rainfall in inches per day in a prescribed area of the rain forest. The square footage of vegetation and relative plant density ...
... 1. Determine whether each of the following is an example of observation and data, a theory, a hypothesis, a control, or a model. a. A research team records the rainfall in inches per day in a prescribed area of the rain forest. The square footage of vegetation and relative plant density ...
Chapter 3
... the explosive combination of hydrogen and oxygen gases to produce water. A chemical equation uses chemical symbols to denote what occurs in a chemical reaction. We have seen how chemists represent elements and compounds using chemical symbols. Now we will look at how chemists represent chemical reac ...
... the explosive combination of hydrogen and oxygen gases to produce water. A chemical equation uses chemical symbols to denote what occurs in a chemical reaction. We have seen how chemists represent elements and compounds using chemical symbols. Now we will look at how chemists represent chemical reac ...
OCR A Level Chemistry A H432 Specification
... Teaching of practical skills is integrated with the theoretical topics and they’re assessed both through written papers and, for A level only, the Practical Endorsement. Chemistry B (Salters) – a context-led approach. Learners study chemistry in a range of different contexts, conveying the excitemen ...
... Teaching of practical skills is integrated with the theoretical topics and they’re assessed both through written papers and, for A level only, the Practical Endorsement. Chemistry B (Salters) – a context-led approach. Learners study chemistry in a range of different contexts, conveying the excitemen ...
B.Sc. (Hons.) Chemistry
... der Waals equation of state, its derivation and application in explaining real gas behaviour, mention of other equations of state (Berthelot, Dietrici); virial equation of state; van der Waals equation expressed in virial form and calculation of Boyle temperature. Isotherms of real gases and their ...
... der Waals equation of state, its derivation and application in explaining real gas behaviour, mention of other equations of state (Berthelot, Dietrici); virial equation of state; van der Waals equation expressed in virial form and calculation of Boyle temperature. Isotherms of real gases and their ...
A novel process of dye wastewater treatment by linking advanced
... problems to be solved in industry, which may have a long-term negative impact on ecosystems when directly discharged into water bodies. Due to the existence of complicated chemical compounds (such as aromatic amines and nitroanilines) in dye wastewaters, it is commonly considered to be of very low b ...
... problems to be solved in industry, which may have a long-term negative impact on ecosystems when directly discharged into water bodies. Due to the existence of complicated chemical compounds (such as aromatic amines and nitroanilines) in dye wastewaters, it is commonly considered to be of very low b ...
Novel Methods and Materials in Development of Liquid Carrier
... using ionic liquids in membrane separations and provided them to us directly from his labs. Without him the thesis would definitely concern another research topic. Some aspects of my work were treated by the students of chemical engineering at IVT that I supervised as good as I could. In spite of ne ...
... using ionic liquids in membrane separations and provided them to us directly from his labs. Without him the thesis would definitely concern another research topic. Some aspects of my work were treated by the students of chemical engineering at IVT that I supervised as good as I could. In spite of ne ...
Chemistry - Department of Education and Skills
... improved quality of drinking water and food products, etc. The individual modules making up this handbook have been selected around the content and structure of the syllabus to provide easy access to resource material in a way which supports the implementation of the course. However, it is important ...
... improved quality of drinking water and food products, etc. The individual modules making up this handbook have been selected around the content and structure of the syllabus to provide easy access to resource material in a way which supports the implementation of the course. However, it is important ...
sample
... A) molecules in the gas phase are in constant motion. B) molecules in the gas phase have more potential energy than in solids. C) molecules in the gas phase have more kinetic energy than in solids. D) gaseous molecules have less mass. E) molecules in the gas phase have more space between them than i ...
... A) molecules in the gas phase are in constant motion. B) molecules in the gas phase have more potential energy than in solids. C) molecules in the gas phase have more kinetic energy than in solids. D) gaseous molecules have less mass. E) molecules in the gas phase have more space between them than i ...
Westwood High School Lesson Plans
... o Why is one can floating and the other sinking? One can is floating and the other is not because while they both have the same volume, one can weighs more than the other due to the sugar content, making it more dense than the other can. o What are some things that would contribute to the one can ...
... o Why is one can floating and the other sinking? One can is floating and the other is not because while they both have the same volume, one can weighs more than the other due to the sugar content, making it more dense than the other can. o What are some things that would contribute to the one can ...
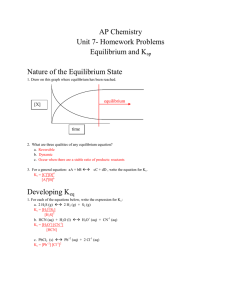
AP Chemistry Unit 7- Homework Problems Equilibrium and Ksp
... 1. Will a ppt of CaCO3 (Ksp= 3.4 x10-9) form if [Ca+2] = 4 x10-6 M and [CO3-2] = 4 x10-3? Q = [4 x10-6][4x10-3] = 1.6x10-8 >> 3.4x10-9 so yes, ppt 2. Will a ppt of Ag2CrO4 (Ksp = 1.1 x10-12 ) form if [Ag+] = 3x10-4 and [CrO4-2] = 2x10-4? Q = [3 x10-4]2 [2x10-4] = 1.8x10-11 >> 1.1x10-12 so yes, ppt 3 ...
... 1. Will a ppt of CaCO3 (Ksp= 3.4 x10-9) form if [Ca+2] = 4 x10-6 M and [CO3-2] = 4 x10-3? Q = [4 x10-6][4x10-3] = 1.6x10-8 >> 3.4x10-9 so yes, ppt 2. Will a ppt of Ag2CrO4 (Ksp = 1.1 x10-12 ) form if [Ag+] = 3x10-4 and [CrO4-2] = 2x10-4? Q = [3 x10-4]2 [2x10-4] = 1.8x10-11 >> 1.1x10-12 so yes, ppt 3 ...
Chapter 8: Balances on Nonreactive Processes
... m3 and must be cooled down to a temperature of 30°C before being fed to a proton exchange membrane fuel cell. Determine the amount of heat required to cool down the hydrogen obtained from the steam-methane reforming process. Assume that the constant-volume heat capacity of hydrogen is given by the f ...
... m3 and must be cooled down to a temperature of 30°C before being fed to a proton exchange membrane fuel cell. Determine the amount of heat required to cool down the hydrogen obtained from the steam-methane reforming process. Assume that the constant-volume heat capacity of hydrogen is given by the f ...
Thermodynamics and Phase Diagrams
... fundamental equation of thermodynamics. We have assumed that the only work term is the reversible work of expansion (sometimes called “PV work”.) In general, in this chapter, this will be the case. ...
... fundamental equation of thermodynamics. We have assumed that the only work term is the reversible work of expansion (sometimes called “PV work”.) In general, in this chapter, this will be the case. ...
Oxidation of Reduced Sulfur Species: Carbon
... characterized using the W1U method43 as implemented in the Gaussian 09 program.44 We modified this method to base it on geometry optimization and vibrational frequencies at the QCISD/6-311G(2d,d,p) level of theory, followed by several component steps that are combined to yield an approximate coupled ...
... characterized using the W1U method43 as implemented in the Gaussian 09 program.44 We modified this method to base it on geometry optimization and vibrational frequencies at the QCISD/6-311G(2d,d,p) level of theory, followed by several component steps that are combined to yield an approximate coupled ...
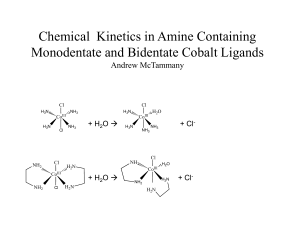
Chemical Kinetics in Monodentate and Bidentate Cobalt Compounds
... [Co(NH3)4CO3]NO3 is first produced as an intermediate by adding (NH4)2 CO3 to Cobalt (II) Nitrate hexahydrate in concentrated ammonia. 30% hydrogen peroxide was then added slowly to the precipitate. NH3, (NH4)2 CO3 , H2O2 ...
... [Co(NH3)4CO3]NO3 is first produced as an intermediate by adding (NH4)2 CO3 to Cobalt (II) Nitrate hexahydrate in concentrated ammonia. 30% hydrogen peroxide was then added slowly to the precipitate. NH3, (NH4)2 CO3 , H2O2 ...
Effect of alkali species on synthesis of KF zeolitic materials
... the sum of released amounts of K+ and Na+. The amount of Ca2+ released gradually increased to 500 cmol/kg and decreased to 300 cmol/kg at Na/(Na + K) = 1, with increasing Na/(Na + K) ratios in the mixed solution, which mean that the product synthesized in mixed solution contains higher releasable Ca ...
... the sum of released amounts of K+ and Na+. The amount of Ca2+ released gradually increased to 500 cmol/kg and decreased to 300 cmol/kg at Na/(Na + K) = 1, with increasing Na/(Na + K) ratios in the mixed solution, which mean that the product synthesized in mixed solution contains higher releasable Ca ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.