Chapter 1: Chemistry: The Study of Change
... 6. Which of the following is the correct net ionic equation for the reaction that occurs when solutions of Pb(NO3)2 and NH4Cl are mixed? A) Pb(NO3)2(aq) + 2NH4Cl(aq) NH4NO3(aq) + PbCl2(s) B) Pb2+(aq) + 2Cl–(aq) PbCl2(s) C) Pb2+(aq) + 2NO3– (aq) + 2NH 4 (aq) + 2Cl–(aq) 2NH 4 (aq) + 2NO3– (aq) ...
... 6. Which of the following is the correct net ionic equation for the reaction that occurs when solutions of Pb(NO3)2 and NH4Cl are mixed? A) Pb(NO3)2(aq) + 2NH4Cl(aq) NH4NO3(aq) + PbCl2(s) B) Pb2+(aq) + 2Cl–(aq) PbCl2(s) C) Pb2+(aq) + 2NO3– (aq) + 2NH 4 (aq) + 2Cl–(aq) 2NH 4 (aq) + 2NO3– (aq) ...
CUCURBIT[7]URIL HOST-GUEST COMPLEXES WITH DRUG MOLECULES CONTAINING ISOQUINOLINE GROUPS Julian Kwok by
... the largest when the bridge is two carbons long. This is due to the two isoquinolinium groups being in close proximity to each other, allowing CB[7] to interact with both nitrogens while still binding around the isoquinoline group. ...
... the largest when the bridge is two carbons long. This is due to the two isoquinolinium groups being in close proximity to each other, allowing CB[7] to interact with both nitrogens while still binding around the isoquinoline group. ...
Homework1-4-Answers
... following chemical equation? (Section: 3.9) 2P(s) + 3I2(s) 2PI3(s) Ans: I2 24. Oxidation of a hydrocarbon gave a product composed of carbon, hydrogen, and oxygen. The product that was purified and sent off for elemental analysis giving the following mass percents: 68.85% C and 4.95% H. Determine t ...
... following chemical equation? (Section: 3.9) 2P(s) + 3I2(s) 2PI3(s) Ans: I2 24. Oxidation of a hydrocarbon gave a product composed of carbon, hydrogen, and oxygen. The product that was purified and sent off for elemental analysis giving the following mass percents: 68.85% C and 4.95% H. Determine t ...
10. Solution Guide to Supplementary Exercises
... Each question (Questions 56 – 60) consists of two separate statements. Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the fo ...
... Each question (Questions 56 – 60) consists of two separate statements. Decide whether each of the two statements is true or false; if both are true, then decide whether or not the second statement is a correct explanation of the first statement. Then select one option from A to D according to the fo ...
Oxygen Isotope Effects as Structural and Mechanistic Probes in
... active sites and, as a result, the significance of the findings concentrated on the observation that living organisms were capable of fractionating oxygen isotopes in the atmosphere, causing the 18O/16O to differ from that of ocean water. Later measurements from the plant biology community suggested ...
... active sites and, as a result, the significance of the findings concentrated on the observation that living organisms were capable of fractionating oxygen isotopes in the atmosphere, causing the 18O/16O to differ from that of ocean water. Later measurements from the plant biology community suggested ...
syllabus for two‐year four‐semester course in chemistry 2014
... MO treatment of acyclic and cyclic conjugated systems; Huckel’s rule and concept of aromaticty, annulenes, heteroannulenes, fullerenes (C60), alternate and non-alternate hydrocarbons, anti-aromaticity, pseudo-aromaticity, homo-aromaticity; graphical methodsFrost diagram. Huckel treatment – applicati ...
... MO treatment of acyclic and cyclic conjugated systems; Huckel’s rule and concept of aromaticty, annulenes, heteroannulenes, fullerenes (C60), alternate and non-alternate hydrocarbons, anti-aromaticity, pseudo-aromaticity, homo-aromaticity; graphical methodsFrost diagram. Huckel treatment – applicati ...
Unit 3 2 Basic Mole Conversions and Mole Maps
... Meaning: For every 2 mol of ethane, 7 mol of dioxygen molecules are consumed in the combustion (a ratio of 2 to 7) This produces 4 moles of carbon dioxide, 6 moles of water and releases 3,170 kJ of energy from the bonding chemicals to the environment. This set of relationships can indicate, at a gla ...
... Meaning: For every 2 mol of ethane, 7 mol of dioxygen molecules are consumed in the combustion (a ratio of 2 to 7) This produces 4 moles of carbon dioxide, 6 moles of water and releases 3,170 kJ of energy from the bonding chemicals to the environment. This set of relationships can indicate, at a gla ...
Lecture notes
... Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, Chemistry, 2012 (John Wiley) ISBN: 978 1 74246 707 8 ...
... Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, Chemistry, 2012 (John Wiley) ISBN: 978 1 74246 707 8 ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Zn (s) + 2 HCl (aq) ZnCl2 (aq) + H2 (g) Zn (s) + 2 CuNO3 (aq) 2 Cu (s) + Zn(NO3)2 (aq) Cu (s) + 2 AgNO3 (aq) 2 Ag (s) + Cu(NO3)2 (aq) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) ...
... Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Zn (s) + 2 HCl (aq) ZnCl2 (aq) + H2 (g) Zn (s) + 2 CuNO3 (aq) 2 Cu (s) + Zn(NO3)2 (aq) Cu (s) + 2 AgNO3 (aq) 2 Ag (s) + Cu(NO3)2 (aq) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) ...
Vol 1 No 2.10
... state, R (J.mol-1. K-1) is the universal gas constant, µ0 (J.mol-1) is the chemical potential at the pressure P0 and temperature T, and ϑ (m3/mol) is the partial molar volume of the species. According to the Eq. (2), under steady state flow, the chemical potential decreases linearly, and the concent ...
... state, R (J.mol-1. K-1) is the universal gas constant, µ0 (J.mol-1) is the chemical potential at the pressure P0 and temperature T, and ϑ (m3/mol) is the partial molar volume of the species. According to the Eq. (2), under steady state flow, the chemical potential decreases linearly, and the concent ...
Surface and sub-surface reactions during low temperature
... addition, surface reaction control can also enable direct formation of useful structures. For example, patterns of self-assembled organic monolayers with designed surface termination can spatially define sites to promote12 or impede13–15 film nucleation and growth, producing self-patterned films for ...
... addition, surface reaction control can also enable direct formation of useful structures. For example, patterns of self-assembled organic monolayers with designed surface termination can spatially define sites to promote12 or impede13–15 film nucleation and growth, producing self-patterned films for ...
Unit 5 Organic Chemistry
... reactions of photosynthesis, digestion, and respiration (Figure 1). The formation of fossil fuels can be seen as the end of the natural processes in the carbon cycle and the beginning of the technological processes described in this chapter. Humans have invented methods to extract fossil fuels from ...
... reactions of photosynthesis, digestion, and respiration (Figure 1). The formation of fossil fuels can be seen as the end of the natural processes in the carbon cycle and the beginning of the technological processes described in this chapter. Humans have invented methods to extract fossil fuels from ...
Rates of Reaction
... – A more useful mathematical relationship would show how a reactant concentration changes over a period of time. ...
... – A more useful mathematical relationship would show how a reactant concentration changes over a period of time. ...
2016 Product Catalogue
... (Identifying Information), is strictly confidential, except as detailed below. RCPAQAP keeps all participant details confidential. Such details will not be disclosed to a third party, unless required by legislation, without the prior written consent of the participant. In the course of administering ...
... (Identifying Information), is strictly confidential, except as detailed below. RCPAQAP keeps all participant details confidential. Such details will not be disclosed to a third party, unless required by legislation, without the prior written consent of the participant. In the course of administering ...
M - coercingmolecules
... by counting or weighing them, depending on which method is more convenient ...
... by counting or weighing them, depending on which method is more convenient ...
2017 Product Catalogue
... Purchasing a Data Management enrolment is useful when the material from the Full Program can be shared with a second instrument or a satellite/branch laboratory. NOTE: A Participant Number may have either a Full Program OR a Data Management enrolment, NOT BOTH Modules All Chemical Pathology modules ...
... Purchasing a Data Management enrolment is useful when the material from the Full Program can be shared with a second instrument or a satellite/branch laboratory. NOTE: A Participant Number may have either a Full Program OR a Data Management enrolment, NOT BOTH Modules All Chemical Pathology modules ...
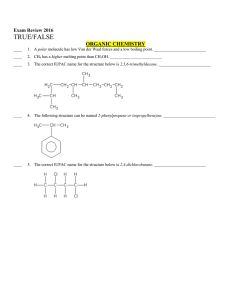
Multiple Choice Exam Review June 2016
... ____ 12. The shape of SO2 is trigonal planar. ____________________ ____ 13. The valence p orbitals in phosphorus, P, are half-filled. ____________________ ____ 14. All of the valence electrons in Fe2+ must have the same spin. _________________________ ____ 15. The shape of boron trifluoride, BF3, is ...
... ____ 12. The shape of SO2 is trigonal planar. ____________________ ____ 13. The valence p orbitals in phosphorus, P, are half-filled. ____________________ ____ 14. All of the valence electrons in Fe2+ must have the same spin. _________________________ ____ 15. The shape of boron trifluoride, BF3, is ...
Chemistry XII - Kendriya Vidyalaya IIM,Lucknow
... Ans : Q = it = 5 Х 2 Х 60 Х 60 = 3600 C No. of electrons flow = 3600 Х 6.022 Х 1023 ...
... Ans : Q = it = 5 Х 2 Х 60 Х 60 = 3600 C No. of electrons flow = 3600 Х 6.022 Х 1023 ...
Section – B - About iTutoring
... The equilibrium established in chemical reactions is called chemical equilibrium. (3). How can be said that equilibrium is dynamic in nature? The equilibrium is dynamic and not steady as the forward and the reverse reactions occur with the same velocity at the equilibrium time in equilibrium reactio ...
... The equilibrium established in chemical reactions is called chemical equilibrium. (3). How can be said that equilibrium is dynamic in nature? The equilibrium is dynamic and not steady as the forward and the reverse reactions occur with the same velocity at the equilibrium time in equilibrium reactio ...
KCl + O KClO 3 → However, this equation is not balanced, since
... CHEMICAL EQUATIONS, AND CALCULATIONS FROM THEM ...
... CHEMICAL EQUATIONS, AND CALCULATIONS FROM THEM ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.

![CUCURBIT[7]URIL HOST-GUEST COMPLEXES WITH DRUG MOLECULES CONTAINING ISOQUINOLINE GROUPS Julian Kwok by](http://s1.studyres.com/store/data/008101179_1-fa974bb5e0d463f251947f4fb85d5098-300x300.png)