TEKS 5 - Online Learning Exchange
... Because the actual yield of a chemical reaction is often less than the theoretical yield, the percent yield is often less than 100 percent. The percent yield is a measure of the efficiency of a reaction carried out yield is similar to an exam score measuring your efficiency of learning or a in the ...
... Because the actual yield of a chemical reaction is often less than the theoretical yield, the percent yield is often less than 100 percent. The percent yield is a measure of the efficiency of a reaction carried out yield is similar to an exam score measuring your efficiency of learning or a in the ...
Changing Matter
... Chemical Changes • To show conservation of mass Balance equations – Make sure there are the same number of each type of atom in the products and in the reactants ...
... Chemical Changes • To show conservation of mass Balance equations – Make sure there are the same number of each type of atom in the products and in the reactants ...
The science of chemistry is concerned
... The chemical reaction in this example is of environmental interest. Iron pyrite (FeS2 ) is often an impurity in coal, and so burning this fuel in a power plant produces sulfur dioxide ( SO 2 ), a major air pollutant. Our next example also involves burning a fuel and its effect on the atmosphere. ...
... The chemical reaction in this example is of environmental interest. Iron pyrite (FeS2 ) is often an impurity in coal, and so burning this fuel in a power plant produces sulfur dioxide ( SO 2 ), a major air pollutant. Our next example also involves burning a fuel and its effect on the atmosphere. ...
Calculations with Chemical Formulas and Equations
... The trick: • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... The trick: • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
5 Steps
... the exam. These steps will provide you with the skills and strategies vital to the exam, and the practice that will lead you to towards the perfect 5. First, we start by introducing the basic five-step plan used in this book. Then in Chapter 1, we will give you some background information about the ...
... the exam. These steps will provide you with the skills and strategies vital to the exam, and the practice that will lead you to towards the perfect 5. First, we start by introducing the basic five-step plan used in this book. Then in Chapter 1, we will give you some background information about the ...
UNIVERSITY OF DELHI FACULTY OF SCIENCE SYLLABUS OF COURSES TO BE OFFERED
... students in the examinations, the UGC has formulated these guidelines. ...
... students in the examinations, the UGC has formulated these guidelines. ...
Hidden Scale Invariance in Condensed Matter
... A. Three States of Matter. Matter comes in three states: gas, liquid, and solid.1,2 The gas state is the simplest. Here its constituents move in straight lines most of the time, interrupted by violent collisions. This fact is responsible for a gas’ structure, dynamics, and thermodynamics. If the gas ...
... A. Three States of Matter. Matter comes in three states: gas, liquid, and solid.1,2 The gas state is the simplest. Here its constituents move in straight lines most of the time, interrupted by violent collisions. This fact is responsible for a gas’ structure, dynamics, and thermodynamics. If the gas ...
Chapter 3
... In Chapter 2, we saw the importance of relative numbers of atoms in the formation of compounds. We also learned how relative masses of atoms can be based on the arbitrary choice of the carbon-12 atom as a standard (Section 2.4). Now, we introduce a concept that enables us to deal with actual rather ...
... In Chapter 2, we saw the importance of relative numbers of atoms in the formation of compounds. We also learned how relative masses of atoms can be based on the arbitrary choice of the carbon-12 atom as a standard (Section 2.4). Now, we introduce a concept that enables us to deal with actual rather ...
Solutions to Exercises
... P4O10 and SO2. In principle, you could calculate Hrxn from enthalpies of formation for the reactants and products of this reaction. You would require the values for P 4S3, O2 (which equals zero), P4O10, and SO2. Enthalpies of formation for the products, P4O10 and SO2, are given in Table 6.2 in the ...
... P4O10 and SO2. In principle, you could calculate Hrxn from enthalpies of formation for the reactants and products of this reaction. You would require the values for P 4S3, O2 (which equals zero), P4O10, and SO2. Enthalpies of formation for the products, P4O10 and SO2, are given in Table 6.2 in the ...
Adsorption and desorption
... γf,n free energy of adlayer-film of thickness n; γin free energy of interface; ...
... γf,n free energy of adlayer-film of thickness n; γin free energy of interface; ...
5 Steps to a 5 AP Chemistry, 2008-2009 Edition
... Welcome to the AP Chemistry Five-Step Program. The fact that you are reading this preface suggests that you will be taking the AP exam in chemistry. The AP Chemistry exam is constantly evolving and so this guide has evolved. In this edition, we have updated the book to match the new AP Chemistry exa ...
... Welcome to the AP Chemistry Five-Step Program. The fact that you are reading this preface suggests that you will be taking the AP exam in chemistry. The AP Chemistry exam is constantly evolving and so this guide has evolved. In this edition, we have updated the book to match the new AP Chemistry exa ...
Chemistry
... Behaviour of real gases: Deviations from ideal gas behaviour, compressibility factor, Z, and its variation with pressure and temperature for different gases. Causes of deviation from ideal behaviour. van der Waals equation of state, its derivation and application in explaining real gas behaviour, ca ...
... Behaviour of real gases: Deviations from ideal gas behaviour, compressibility factor, Z, and its variation with pressure and temperature for different gases. Causes of deviation from ideal behaviour. van der Waals equation of state, its derivation and application in explaining real gas behaviour, ca ...
Calculations with Chemical Formulas and Equations
... The trick: • By definition, this is the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... The trick: • By definition, this is the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Chapter 3 Stoichiometry: Calculations with Chemical
... The trick: • By definition, this is the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... The trick: • By definition, this is the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and
... The trick: • By definition, this is the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... The trick: • By definition, this is the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
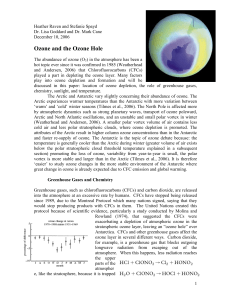
Ozone Writeup - International Research Institute for Climate and
... current studies use new estimates of equivalent effective Antarctic stratospheric chlorine in their model runs. The Newman et al. (2006) study also predicts that ozone hole recovery will first be statistically detectable in about 2024. In contradiction to studies that predict a date for recovery, W ...
... current studies use new estimates of equivalent effective Antarctic stratospheric chlorine in their model runs. The Newman et al. (2006) study also predicts that ozone hole recovery will first be statistically detectable in about 2024. In contradiction to studies that predict a date for recovery, W ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.