Solutions Manual
... © Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006. This page from Chemistry Contexts 2 Teacher’s Resource second edition may be reproduced for classroom use. ...
... © Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006. This page from Chemistry Contexts 2 Teacher’s Resource second edition may be reproduced for classroom use. ...
General and Inorganic Chemistry – Laboratory Techniques
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
Rh(acac)(CO)(PR1R2R3) - University of the Free State
... Rhodium is often used as an alloying agent to harden platinum and palladium. It is used in electrical contact material, due to its low electrical resistance, and in optical instruments and jewellery because of its high reflectance and hardness. It is extensively used in chemical synthesis as an impo ...
... Rhodium is often used as an alloying agent to harden platinum and palladium. It is used in electrical contact material, due to its low electrical resistance, and in optical instruments and jewellery because of its high reflectance and hardness. It is extensively used in chemical synthesis as an impo ...
Water Chemistry - U
... the book’s companion Web site at www.oup.com/us/WaterChemistry, which contains downloadable copies of several tables of data, an interface for the kinetics software, Acuchem, additional problems and figures, and other useful information. Patrick L. Brezonik and William A. Arnold University of Minneso ...
... the book’s companion Web site at www.oup.com/us/WaterChemistry, which contains downloadable copies of several tables of data, an interface for the kinetics software, Acuchem, additional problems and figures, and other useful information. Patrick L. Brezonik and William A. Arnold University of Minneso ...
chemistry - Brilliant Public School Sitamarhi
... The well known mineral fluorite is chemically calcium fluoride. It is known that in one unit cell of this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell ...
... The well known mineral fluorite is chemically calcium fluoride. It is known that in one unit cell of this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell ...
Calculations and the Chemical Equation
... products are shown in parentheses. The symbol over the reaction arrow means that heat energy is necessary for the reaction to occur. The equation must be balanced to reflect the law of conservation of mass, which states that matter can neither be gained nor lost in the process of a chemical react ...
... products are shown in parentheses. The symbol over the reaction arrow means that heat energy is necessary for the reaction to occur. The equation must be balanced to reflect the law of conservation of mass, which states that matter can neither be gained nor lost in the process of a chemical react ...
CHEMKIN Tutorials Manual
... 2-12 Axial gas temperature profiles predicted with and without gas radiation heat loss as compared against experimental temperature profile for the phii=0.6 CH4/O2/N2 flame at 14.6 atm. .......................................................46 2-13 Comparisons of measured and predicted NO mole fract ...
... 2-12 Axial gas temperature profiles predicted with and without gas radiation heat loss as compared against experimental temperature profile for the phii=0.6 CH4/O2/N2 flame at 14.6 atm. .......................................................46 2-13 Comparisons of measured and predicted NO mole fract ...
Atomic and electronic structure of MoS2 nanoparticles
... obtain an estimate of the corresponding Helmholtz free energy we assume the modes to be dispersionless and find f vib⬃10⫺2 eV per mode at room temperature. Hence, if the frequencies at the edges are not significantly changed the vibrational contribution will be small and thus Eq. 共5兲 represents a re ...
... obtain an estimate of the corresponding Helmholtz free energy we assume the modes to be dispersionless and find f vib⬃10⫺2 eV per mode at room temperature. Hence, if the frequencies at the edges are not significantly changed the vibrational contribution will be small and thus Eq. 共5兲 represents a re ...
Oxidative Alihatic Carbon-Carbon Bond Cleavage Reactions
... Dr. Lisa Berreau, for her continued advice and support, and for putting up with me telling her she was wrong (even when she was right). I’m especially grateful for the freedom she has given me in pursuing chemistry that is inherently interesting, even if it was not always the chemistry we had origin ...
... Dr. Lisa Berreau, for her continued advice and support, and for putting up with me telling her she was wrong (even when she was right). I’m especially grateful for the freedom she has given me in pursuing chemistry that is inherently interesting, even if it was not always the chemistry we had origin ...
Thermal Energy 16.050
... Properties may be extensive or intensive. Extensive properties are additive. Thus, if the system is divided into a number of sub-systems, the value of the property for the whole system is equal to the sum of the values for the parts. Volume is an extensive property. Intensive properties do not depen ...
... Properties may be extensive or intensive. Extensive properties are additive. Thus, if the system is divided into a number of sub-systems, the value of the property for the whole system is equal to the sum of the values for the parts. Volume is an extensive property. Intensive properties do not depen ...
Chemistry Final Exam Review
... ____ 87. Which observation does NOT indicate that a chemical reaction has occurred? a. formation of a precipitate c. evolution of heat and light b. production of a gas d. change in total mass of substances ____ 88. A solid produced by a chemical reaction in solution that separates from the solution ...
... ____ 87. Which observation does NOT indicate that a chemical reaction has occurred? a. formation of a precipitate c. evolution of heat and light b. production of a gas d. change in total mass of substances ____ 88. A solid produced by a chemical reaction in solution that separates from the solution ...
CHAPTER 9
... Antoine Laurent Lavoisier was a meticulous scientist. He realized that Rutherford and Priestley had carefully observed and described their experiments but had not measured the mass of anything. Unlike his colleagues, Lavoisier knew the importance of using a balance. He measured the masses of reactan ...
... Antoine Laurent Lavoisier was a meticulous scientist. He realized that Rutherford and Priestley had carefully observed and described their experiments but had not measured the mass of anything. Unlike his colleagues, Lavoisier knew the importance of using a balance. He measured the masses of reactan ...
Major 01 - KFUPM Faculty List
... Solution of the 1st Major Exam, Term 061, Version 000, all correct choices are A 1. All of the following are properties of sodium. Which one is a physical change? A. It is a solid at 25oC and melts at 98oC. Since melting has no change of the chemical composition involved, this is a physical change. ...
... Solution of the 1st Major Exam, Term 061, Version 000, all correct choices are A 1. All of the following are properties of sodium. Which one is a physical change? A. It is a solid at 25oC and melts at 98oC. Since melting has no change of the chemical composition involved, this is a physical change. ...
SCH4U TEXT BOOK
... chemistry student, you are aware that these labels may be misleading. Are all “chemicals” harmful in food, as some of the current advertising suggests? Many terms are used inaccurately in everyday life. The word “natural” is often used in a manner suggesting that all natural compounds are safe and h ...
... chemistry student, you are aware that these labels may be misleading. Are all “chemicals” harmful in food, as some of the current advertising suggests? Many terms are used inaccurately in everyday life. The word “natural” is often used in a manner suggesting that all natural compounds are safe and h ...
Using cryo-electron microscopy to determine thermodynamic and
... can be used to directly view the different microstructures of most self-assembled surfactant systems to resolutions of about 2 nm. There have been several recent reviews showing the ability of cryo-TEM to image different amphiphilic systems [16 –18]. However, the additional step of quantifying the i ...
... can be used to directly view the different microstructures of most self-assembled surfactant systems to resolutions of about 2 nm. There have been several recent reviews showing the ability of cryo-TEM to image different amphiphilic systems [16 –18]. However, the additional step of quantifying the i ...
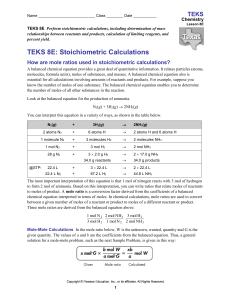
TEKS 5 - Online Learning Exchange
... Because the actual yield of a chemical reaction is often less than the theoretical yield, the percent yield is often less than 100 percent. The percent yield is a measure of the efficiency of a reaction carried out yield is similar to an exam score measuring your efficiency of learning or a in the ...
... Because the actual yield of a chemical reaction is often less than the theoretical yield, the percent yield is often less than 100 percent. The percent yield is a measure of the efficiency of a reaction carried out yield is similar to an exam score measuring your efficiency of learning or a in the ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.