PDF of this page
... Prerequisite: CHEM 3802 with a grade of “C” or better. Computational and modeling software will be introduced through projects involving systems in physical chemistry and spectroscopy as well as organic chemistry, inorganic chemistry, and biochemistry. Computational predictions will be correlated wi ...
... Prerequisite: CHEM 3802 with a grade of “C” or better. Computational and modeling software will be introduced through projects involving systems in physical chemistry and spectroscopy as well as organic chemistry, inorganic chemistry, and biochemistry. Computational predictions will be correlated wi ...
Chemical Properties of Aldehydes and Ketones
... Condensation polymers are substances that are produced when a small molecule such as water is eliminated during polymerization. Polyesters, polyamides, polyurethanes, and phenolics represent four important classes of condensation polymers. ...
... Condensation polymers are substances that are produced when a small molecule such as water is eliminated during polymerization. Polyesters, polyamides, polyurethanes, and phenolics represent four important classes of condensation polymers. ...
Unit 10 complete 2016-2017
... elements can combine in simple whole number ratios to form compounds. Such is the case in the reaction of lead (II) nitrate with sodium iodide. This laboratory investigation will demonstrate this fact and help you understand molar relationships in reactions and their importance in the chemical produ ...
... elements can combine in simple whole number ratios to form compounds. Such is the case in the reaction of lead (II) nitrate with sodium iodide. This laboratory investigation will demonstrate this fact and help you understand molar relationships in reactions and their importance in the chemical produ ...
Honors Chemistry
... elements can combine in simple whole number ratios to form compounds. Such is the case in the reaction of lead (II) nitrate with sodium iodide. This laboratory investigation will demonstrate this fact and help you understand molar relationships in reactions and their importance in the chemical produ ...
... elements can combine in simple whole number ratios to form compounds. Such is the case in the reaction of lead (II) nitrate with sodium iodide. This laboratory investigation will demonstrate this fact and help you understand molar relationships in reactions and their importance in the chemical produ ...
laman web smk raja perempuan, ipoh
... energy changes, principally in the form of heat energy ; the energy changes can be exothermic or endothermic. 2. calculate the heat energy change from experimental measurements using the relationship : energy change = mc∆T 3. define the term enthalphy change of formation, combustion, hydration, solu ...
... energy changes, principally in the form of heat energy ; the energy changes can be exothermic or endothermic. 2. calculate the heat energy change from experimental measurements using the relationship : energy change = mc∆T 3. define the term enthalphy change of formation, combustion, hydration, solu ...
Ch. 12 Stoichiometry
... Step 2: Use the unknown and known in the equation to develop a mole ratio: 2 mol NH3 (unknown from equation) 1 mol N2 (known from equation) ...
... Step 2: Use the unknown and known in the equation to develop a mole ratio: 2 mol NH3 (unknown from equation) 1 mol N2 (known from equation) ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry © 2009, Prentice- ...
... • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry © 2009, Prentice- ...
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and
... • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry 2009, Prentice-H ...
... • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry 2009, Prentice-H ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry © 2009, Prentice- ...
... • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry © 2009, Prentice- ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry 2009, Prentice-H ...
... • By definition, a molar mass is the mass of 1 mol of a substance (i.e., g/mol). – The molar mass of an element is the mass number for the element that we find on the periodic table. – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol). Stoichiometry 2009, Prentice-H ...
Module 1 Predictor Questions
... Using Chemical Formulas to Determine Numbers of Atoms in One Mole of a Substance All of the formulas and symbols introduced up to now can also be used to represent moles of a species. Thus, if asked how many atoms, ions, or molecules there are in one mole of each of these species, simply multiply th ...
... Using Chemical Formulas to Determine Numbers of Atoms in One Mole of a Substance All of the formulas and symbols introduced up to now can also be used to represent moles of a species. Thus, if asked how many atoms, ions, or molecules there are in one mole of each of these species, simply multiply th ...
Chemistry - A Quantitative Science
... 6.0221x1023 = NA is Avogadro's number. A mole is used to indicate a number of atoms just as a dozen is used to indicate a number of eggs. Converting from moles to atoms is done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 at ...
... 6.0221x1023 = NA is Avogadro's number. A mole is used to indicate a number of atoms just as a dozen is used to indicate a number of eggs. Converting from moles to atoms is done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 at ...
29 Sept 08 - Seattle Central
... Reactants and products must occur in numbers that give the same number of each type of atom on both sides of the arrow. ...
... Reactants and products must occur in numbers that give the same number of each type of atom on both sides of the arrow. ...
course file
... Lecture #6 Definition of pure substance, phases: solids liquid and gases, principal phase and sub-phases, Demonstration of mechanical boiling, Introduction to phase diagrams, T-v diagram, Saturation pressure and saturation temperature, Sensible heating, Latent heat of vaporization, Compressed or su ...
... Lecture #6 Definition of pure substance, phases: solids liquid and gases, principal phase and sub-phases, Demonstration of mechanical boiling, Introduction to phase diagrams, T-v diagram, Saturation pressure and saturation temperature, Sensible heating, Latent heat of vaporization, Compressed or su ...
updated chem cp final review key
... f. Adding an enzyme Increases rate of reaction g. Breaking a reactant into smaller pieces Increases rate of reaction 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop ...
... f. Adding an enzyme Increases rate of reaction g. Breaking a reactant into smaller pieces Increases rate of reaction 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop ...
STUDY OF ELECTRODE KINETICS AND THERMODYNAMIC PARAMETERS OF Research Article
... state is accompanied by increase of entropy. From table (4) it can be concluded that as we increase the temperature values of the ΔG# increases and that of the ΔS# decreases continuously, it shows that the non spontaneity of electrode process increases with ...
... state is accompanied by increase of entropy. From table (4) it can be concluded that as we increase the temperature values of the ΔG# increases and that of the ΔS# decreases continuously, it shows that the non spontaneity of electrode process increases with ...
Document
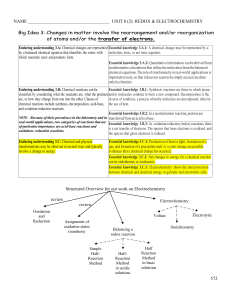
... Essential knowledge 3.A.2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done onl ...
... Essential knowledge 3.A.2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done onl ...
Chapter 6
... row as reactants, and those on the right side of the equilibrium arrow as products. As Berthollet discovered, writing a reaction in this fashion does not guarantee that the reaction of A and B to produce C and D is favorable. Depending on initial conditions, the reaction may move to the left, move t ...
... row as reactants, and those on the right side of the equilibrium arrow as products. As Berthollet discovered, writing a reaction in this fashion does not guarantee that the reaction of A and B to produce C and D is favorable. Depending on initial conditions, the reaction may move to the left, move t ...
Mole-mole factor
... A balanced chemical equations tell us: – The formulas and symbols of the reactants and products – The physical state of each substance – If special conditions such as heat are required – The number of molecules, formula units, or atoms of each type of molecule involved in the reaction • Number can b ...
... A balanced chemical equations tell us: – The formulas and symbols of the reactants and products – The physical state of each substance – If special conditions such as heat are required – The number of molecules, formula units, or atoms of each type of molecule involved in the reaction • Number can b ...
Document
... Essential knowledge 3.A.2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done onl ...
... Essential knowledge 3.A.2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done onl ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.