Origin and metamorphic evolution of magnesite
... Granite forms small bodies cropping out at several localities in the Gemericum. The presence of large volumes of granite at depth was confirmed by numerous boreholes and geophysical measurements (Plančar et al. 1977). In the Dlhá Dolina Valley, the granite is not exposed on the surface, but was pene ...
... Granite forms small bodies cropping out at several localities in the Gemericum. The presence of large volumes of granite at depth was confirmed by numerous boreholes and geophysical measurements (Plančar et al. 1977). In the Dlhá Dolina Valley, the granite is not exposed on the surface, but was pene ...
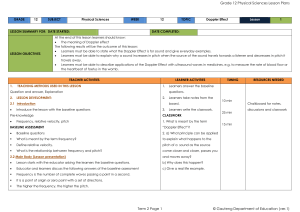
Physical Sciences Grade 12 Term 2
... The siren of a burglar alarm system has a frequency of 960 Hz. During a patrol, a security officer, travelling in his car, hears the siren of the alarm of a house and approaches the house at 2.1 Name the phenomenon that explains the change in the observed frequency. 2.2 Calculate the speed at which ...
... The siren of a burglar alarm system has a frequency of 960 Hz. During a patrol, a security officer, travelling in his car, hears the siren of the alarm of a house and approaches the house at 2.1 Name the phenomenon that explains the change in the observed frequency. 2.2 Calculate the speed at which ...
chemistry - The Aga Khan University
... Kinetic Molecular Theory of Gases 4.1.1 Postulates of Kinetic Molecular Theory 4.1.2 Pressure and Its Units Absolute Temperature Scale on the Basis of Charles Law 4.2.1 Brief recall of Boyle’s and Charles’ Law 4.2.2 Graphical Explanation of Absolute Zero Avogadro’s Law ...
... Kinetic Molecular Theory of Gases 4.1.1 Postulates of Kinetic Molecular Theory 4.1.2 Pressure and Its Units Absolute Temperature Scale on the Basis of Charles Law 4.2.1 Brief recall of Boyle’s and Charles’ Law 4.2.2 Graphical Explanation of Absolute Zero Avogadro’s Law ...
Solutions - ChemConnections
... reason for the sign change in the Ka value, between HF versus HCl, HBr, and HI is entropy. ∆S for the dissociation of HF is very large and negative. There is a high degree of ordering that occurs as the water molecules associate (hydrogen bond) with the small F− ions. The entropy of hydration strong ...
... reason for the sign change in the Ka value, between HF versus HCl, HBr, and HI is entropy. ∆S for the dissociation of HF is very large and negative. There is a high degree of ordering that occurs as the water molecules associate (hydrogen bond) with the small F− ions. The entropy of hydration strong ...
IIT-JEE - Brilliant Public School Sitamarhi
... Crystal defects: Point defects: When ions or atoms do not hold the theoretical position, this is called point defect. Point defects are of two types: Stoichiometric defects: Schottky defect: Due to missing of ions from lattice point in pairs. Frenkel defect: It is caused due to the creation of latti ...
... Crystal defects: Point defects: When ions or atoms do not hold the theoretical position, this is called point defect. Point defects are of two types: Stoichiometric defects: Schottky defect: Due to missing of ions from lattice point in pairs. Frenkel defect: It is caused due to the creation of latti ...
CHEMISTRY
... An introduction to theory and practice of organic chemistry through study of structural principles, reaction mechanisms, and synthesis leading toward end of second term, when complex molecules of biological interest are discussed. Basic goals of course are to develop appreciation and skill in method ...
... An introduction to theory and practice of organic chemistry through study of structural principles, reaction mechanisms, and synthesis leading toward end of second term, when complex molecules of biological interest are discussed. Basic goals of course are to develop appreciation and skill in method ...
jee main-2015 question paper, key & solutions
... Resultant frequency of signal if given by fC f M , f R , f C f M Then possible frequencies are 2005 kHz , 2000 kGz ,1995 kHz An LCR circuit is equivalent to a damped pendulum. In an LCR circuit the capacitor is charged to Q0 and then connected the L and R as shown below: ...
... Resultant frequency of signal if given by fC f M , f R , f C f M Then possible frequencies are 2005 kHz , 2000 kGz ,1995 kHz An LCR circuit is equivalent to a damped pendulum. In an LCR circuit the capacitor is charged to Q0 and then connected the L and R as shown below: ...
Geochemical weathering at the bed of Haut Glacier d`Arolla
... to reconstruct the more complex aspects of subglacial drainage systems that have been revealed by recent borehole investigations (Fountain, 1994; Hubbard et al., 1995; Gordon et al., 1998) and the chemical reactions which might occur within them, without recourse to the direct measurement of the hyd ...
... to reconstruct the more complex aspects of subglacial drainage systems that have been revealed by recent borehole investigations (Fountain, 1994; Hubbard et al., 1995; Gordon et al., 1998) and the chemical reactions which might occur within them, without recourse to the direct measurement of the hyd ...
the chemical and physical properties of condensed
... as phase diagram entities are of this type3032. Yet another type of transformation occurs during the dehydration of a crystalline hydrate. In most systems the phosphate anion is dehydrated without degradation, while in other systems the phosphate anion is destroyed33. Examples of salts which dehydra ...
... as phase diagram entities are of this type3032. Yet another type of transformation occurs during the dehydration of a crystalline hydrate. In most systems the phosphate anion is dehydrated without degradation, while in other systems the phosphate anion is destroyed33. Examples of salts which dehydra ...
Science 9 Year End Review The following information includes all
... _______________ can be used to power entire cities. A DC _______________ is also called a dynamo. It sends current in only one direction because of how the armature is connected to a split ring commutator. An electric motor is constructed in the same way was a generator, but instead it takes ___ ...
... _______________ can be used to power entire cities. A DC _______________ is also called a dynamo. It sends current in only one direction because of how the armature is connected to a split ring commutator. An electric motor is constructed in the same way was a generator, but instead it takes ___ ...
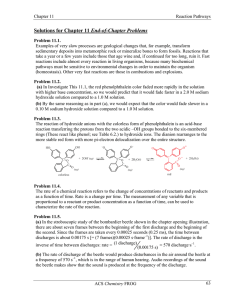
Chapter 6 Chemical Reactions
... should speed up as temperature increases. (As we will find in Section 11.6, this is a relatively minor effect compared to the activation energy effect, but even reactions with zero activation energy go faster at higher temperature because of the faster motion.) Problem 11.9. To test whether a substa ...
... should speed up as temperature increases. (As we will find in Section 11.6, this is a relatively minor effect compared to the activation energy effect, but even reactions with zero activation energy go faster at higher temperature because of the faster motion.) Problem 11.9. To test whether a substa ...
File
... molecules are also present. There is an apparent increase in ordering when these ions are placed in water as compared to the separated state. The hydrating water molecules must be in a highly ordered arrangement when surrounding these anions. G = RTlnK = H TS; HX(aq) ⇌ H+(aq) + X(aq) Ka re ...
... molecules are also present. There is an apparent increase in ordering when these ions are placed in water as compared to the separated state. The hydrating water molecules must be in a highly ordered arrangement when surrounding these anions. G = RTlnK = H TS; HX(aq) ⇌ H+(aq) + X(aq) Ka re ...
2014 Syllabus - Cambridge International Examinations
... construct arguments to support hypotheses or to justify a course of action ...
... construct arguments to support hypotheses or to justify a course of action ...
Enthalpy Barriers for Asymmetric SN2 Alkyl
... alcohols in mixed alcohol systems have been calculated by B3LYP, MP2, and G3(MP2) computational methods. As expected, on the basis of the proton affinities of the alcohols, the enthalpy barrier for transfer of the larger alkyl cation is calculated to be lower than that for transfer of the smaller al ...
... alcohols in mixed alcohol systems have been calculated by B3LYP, MP2, and G3(MP2) computational methods. As expected, on the basis of the proton affinities of the alcohols, the enthalpy barrier for transfer of the larger alkyl cation is calculated to be lower than that for transfer of the smaller al ...
Chapter 19 Homework Problems Answers
... to give gaseous atoms, and then allowing the gaseous atoms to form all of the appropriate bonds. The overall enthalpy of formation by this route is numerically equal to that for the above reaction, and, conveniently, the enthalpy changes for each step are available in either Table 19.3 or Table 19.4 ...
... to give gaseous atoms, and then allowing the gaseous atoms to form all of the appropriate bonds. The overall enthalpy of formation by this route is numerically equal to that for the above reaction, and, conveniently, the enthalpy changes for each step are available in either Table 19.3 or Table 19.4 ...
on the Reduction Behaviour of Iron Oxide Using Carbon Monoxide
... Ru-Fe2O3, Os-Fe2O3, Ag-Fe2O3 and undoped Fe2O3 in nonisothermal reduction under 10% CO in nitrogen are shown in Fig. 5. TPR profiles of undoped Fe2O3 displayed a 3 steps reduction mechanism with one reduction peak at lower temperature (361 ⁰C) represent the reduction of Fe2O3 → Fe3O4 and 2 broad pea ...
... Ru-Fe2O3, Os-Fe2O3, Ag-Fe2O3 and undoped Fe2O3 in nonisothermal reduction under 10% CO in nitrogen are shown in Fig. 5. TPR profiles of undoped Fe2O3 displayed a 3 steps reduction mechanism with one reduction peak at lower temperature (361 ⁰C) represent the reduction of Fe2O3 → Fe3O4 and 2 broad pea ...
B.Sc Chemistry - Calicut University
... to the different methodologies used in science.. Therefore, one module each on methodology in science and methodology in chemistry is introduced which helps the student to get an idea on the tactics and strategies to be adopted in chemistry. Here a detailed study is not expected, instead an introduc ...
... to the different methodologies used in science.. Therefore, one module each on methodology in science and methodology in chemistry is introduced which helps the student to get an idea on the tactics and strategies to be adopted in chemistry. Here a detailed study is not expected, instead an introduc ...
AQA A-level Chemistry
... and products in a chemical reaction. The vertical (y) axis is enthalpy but not ∆H. The horizontal (x) axis is progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines are drawn and labelled with the names or formulae of reactants and products. These represent the enthalp ...
... and products in a chemical reaction. The vertical (y) axis is enthalpy but not ∆H. The horizontal (x) axis is progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines are drawn and labelled with the names or formulae of reactants and products. These represent the enthalp ...
PDF of this page
... Prerequisite: CHEM 3802 with a grade of “C” or better. Computational and modeling software will be introduced through projects involving systems in physical chemistry and spectroscopy as well as organic chemistry, inorganic chemistry, and biochemistry. Computational predictions will be correlated wi ...
... Prerequisite: CHEM 3802 with a grade of “C” or better. Computational and modeling software will be introduced through projects involving systems in physical chemistry and spectroscopy as well as organic chemistry, inorganic chemistry, and biochemistry. Computational predictions will be correlated wi ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.