SampleTest3
... ____10. In which of the following processes does the kinetic energy of the water increase? A) water freezes B) steam condenses to liquid C) water evaporates D) more than one response is correct ____11. Which of the following is an exothermic process? A) Sublimation [(s) to (g)] B) melting C) evapor ...
... ____10. In which of the following processes does the kinetic energy of the water increase? A) water freezes B) steam condenses to liquid C) water evaporates D) more than one response is correct ____11. Which of the following is an exothermic process? A) Sublimation [(s) to (g)] B) melting C) evapor ...
Structure of Sodium Chloride
... No 1. The structure of sodium chloride and how salt dissolves in water What happens when salt dissolves in water? Salt is an ionic compound, the lattice is formed with negative and positive ions. When added to water ions detach themselves from the crystal and join loosely to water molecules 1. To d ...
... No 1. The structure of sodium chloride and how salt dissolves in water What happens when salt dissolves in water? Salt is an ionic compound, the lattice is formed with negative and positive ions. When added to water ions detach themselves from the crystal and join loosely to water molecules 1. To d ...
Texture and Structure Lab Lab #2
... Vesicles are small and often drawn out into long tubes because of flowage of high viscosity lava. d. Amygdaloidal Structure: Vesicles are infilled with younger minerals. The filling is called an Amygdule and is composed of quartz, opal, chalcedony and or zeolite minerals. D. Other Features Associate ...
... Vesicles are small and often drawn out into long tubes because of flowage of high viscosity lava. d. Amygdaloidal Structure: Vesicles are infilled with younger minerals. The filling is called an Amygdule and is composed of quartz, opal, chalcedony and or zeolite minerals. D. Other Features Associate ...
Minerals
... Mineral Characteristics First, all minerals are formed by natural processes. These are processes that occur on or inside Earth with no input from humans. For example, salt formed by the natural evaporation of seawater is the mineral halite, but salt formed by evaporation of saltwater solutions in la ...
... Mineral Characteristics First, all minerals are formed by natural processes. These are processes that occur on or inside Earth with no input from humans. For example, salt formed by the natural evaporation of seawater is the mineral halite, but salt formed by evaporation of saltwater solutions in la ...
Crystals
... The overall result of these factors result in low solubility of salts containing two large ions (soft-soft) or two small ions (hard-hard). For salts containing two small ions, especially with the same magnitude of charge, the greater lattice energy dominates, and cannot be easily overcome by the hyd ...
... The overall result of these factors result in low solubility of salts containing two large ions (soft-soft) or two small ions (hard-hard). For salts containing two small ions, especially with the same magnitude of charge, the greater lattice energy dominates, and cannot be easily overcome by the hyd ...
Elements and Minerals
... II. Today: From the “big picture” to the VERY small one! • from large-scale hazards to the scale of atoms • critical for understanding the building blocks of geology • we will not spend as much time on this subject as other introductory geology classes o you will get a good review in the recitation ...
... II. Today: From the “big picture” to the VERY small one! • from large-scale hazards to the scale of atoms • critical for understanding the building blocks of geology • we will not spend as much time on this subject as other introductory geology classes o you will get a good review in the recitation ...
Earth`s Tectonic Plates
... for half of the atoms of a radioactive material to decay into something else. These variables can be related using the following equation, T = (1/λ)ln[1 + (D/P)], where “ln” is the natural logarithm (i.e., log with base e). From this information a graph such as this one can be constructed: ...
... for half of the atoms of a radioactive material to decay into something else. These variables can be related using the following equation, T = (1/λ)ln[1 + (D/P)], where “ln” is the natural logarithm (i.e., log with base e). From this information a graph such as this one can be constructed: ...
Honors Chemistry Review Packet KEY
... 6. Liquids and gases both have an indefinite shape; while the shape of a solid is definite, the shape of a liquid is indefinite. 7. It is reversible because solid mercury can be melted back into a liquid again. 8. Platinum and copper can have the same mass and volume (extensive properties). They can ...
... 6. Liquids and gases both have an indefinite shape; while the shape of a solid is definite, the shape of a liquid is indefinite. 7. It is reversible because solid mercury can be melted back into a liquid again. 8. Platinum and copper can have the same mass and volume (extensive properties). They can ...
3.032 Mechanical Behavior of Materials Fall 2007
... Images removed due to copyright restrictions. Please see: Fig. 1c in Gouldstone, Andrew, et al. "Simulation of Defect Nucleation in a Crystal." Nature 411 (July 2001): 656 and Fig. 1 in Van Vliet, Krystyn J., et al. "Model Experiments for Direct Visualization of Grain Boundary Deformation in Nanocry ...
... Images removed due to copyright restrictions. Please see: Fig. 1c in Gouldstone, Andrew, et al. "Simulation of Defect Nucleation in a Crystal." Nature 411 (July 2001): 656 and Fig. 1 in Van Vliet, Krystyn J., et al. "Model Experiments for Direct Visualization of Grain Boundary Deformation in Nanocry ...
HOMEWORK : CHAPTER 20
... magnesium oxide. [Hint : First convert Mg to Mg(NO3)2. Next, MgO can be obtained by heating Mg(NO3)2] 20.36 The second ionization energy of magnesium is only about twice as great as the first, but the third ionization energy is 10 times as great. Why does it take so much more energy to remove the th ...
... magnesium oxide. [Hint : First convert Mg to Mg(NO3)2. Next, MgO can be obtained by heating Mg(NO3)2] 20.36 The second ionization energy of magnesium is only about twice as great as the first, but the third ionization energy is 10 times as great. Why does it take so much more energy to remove the th ...
Science Notes on Physical and Chemical Properties
... Example – Tear a piece of paper into 10-15 pieces. The shape and size have changed, but its still paper Example – Change of state = physical change…add energy to ice and you get a liquid…add more energy and you get a gas…all physical changes as it is still water Example – Dissolving things is a phys ...
... Example – Tear a piece of paper into 10-15 pieces. The shape and size have changed, but its still paper Example – Change of state = physical change…add energy to ice and you get a liquid…add more energy and you get a gas…all physical changes as it is still water Example – Dissolving things is a phys ...
Characteristic Properties Non-Characteristic Properties
... Characteristic Properties Examples (cont.) Melting Point: The temperature at which a substance changes from a solid to a liquid Boiling Point: The temperature at which a substance changes from a liquid to a gas ...
... Characteristic Properties Examples (cont.) Melting Point: The temperature at which a substance changes from a solid to a liquid Boiling Point: The temperature at which a substance changes from a liquid to a gas ...
Course Pack3 Phase Diagrams
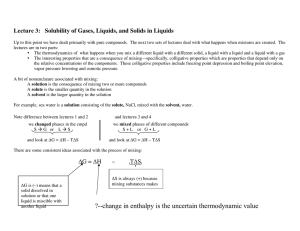
... ∆Hsoln is (+) for NaCl in H2O ∆Hsoln is (–) for Na2SO4 in H2O ∆Hsoln is (–) for O2 in H2O Consider the case that ∆Hmix is negative: since ∆Smix is positive then ∆Gsoln will have to be negative and the reaction happens. Now consider the case that ∆Hmix is positive: in this case the spontaneity of the ...
... ∆Hsoln is (+) for NaCl in H2O ∆Hsoln is (–) for Na2SO4 in H2O ∆Hsoln is (–) for O2 in H2O Consider the case that ∆Hmix is negative: since ∆Smix is positive then ∆Gsoln will have to be negative and the reaction happens. Now consider the case that ∆Hmix is positive: in this case the spontaneity of the ...
Chemistry Lab 2010
... • Saturated: The maximum amount of solute is dissolved in the solvent • Unsaturated: Less than maximum amount of solute is dissolved in the solvent • Supersaturated: More than the maximum amount of solute is dissolved in solvent • To obtain a supersaturated solution, you heat solution until all solu ...
... • Saturated: The maximum amount of solute is dissolved in the solvent • Unsaturated: Less than maximum amount of solute is dissolved in the solvent • Supersaturated: More than the maximum amount of solute is dissolved in solvent • To obtain a supersaturated solution, you heat solution until all solu ...
mineral
... types of atoms that make up a mineral is what makes the mineral unique. The way that the atoms are joined together is also important. ...
... types of atoms that make up a mineral is what makes the mineral unique. The way that the atoms are joined together is also important. ...
CHEM_2nd_Semester_Final_R eview
... 2. Use the information from the previous question to describe the shape and volume for each phase (state) of matter. 3. List the three phases of matter in order of increasing intermolecular attractions. 4. Why do the atoms and molecules in liquids move in a random pattern relative to one another ins ...
... 2. Use the information from the previous question to describe the shape and volume for each phase (state) of matter. 3. List the three phases of matter in order of increasing intermolecular attractions. 4. Why do the atoms and molecules in liquids move in a random pattern relative to one another ins ...
Chemistry 2nd Semester Final Exam Review Chemical Bonds Give
... 2. Use the information from the previous question to describe the shape and volume for each phase (state) of matter. 3. List the three phases of matter in order of increasing intermolecular attractions. 4. Why do the atoms and molecules in liquids move in a random pattern relative to one another ins ...
... 2. Use the information from the previous question to describe the shape and volume for each phase (state) of matter. 3. List the three phases of matter in order of increasing intermolecular attractions. 4. Why do the atoms and molecules in liquids move in a random pattern relative to one another ins ...
2nd Semester Final Review
... 2. Use the information from the previous question to describe the shape and volume for each phase (state) of matter. 3. List the three phases of matter in order of increasing intermolecular attractions. 4. Why do the atoms and molecules in liquids move in a random pattern relative to one another ins ...
... 2. Use the information from the previous question to describe the shape and volume for each phase (state) of matter. 3. List the three phases of matter in order of increasing intermolecular attractions. 4. Why do the atoms and molecules in liquids move in a random pattern relative to one another ins ...
High-Throughput Low-Volume Protein Crystallization
... which cost hundreds of thousands of dollars and are prone to mechanical problems, can only pipette down to 20 nanoliter. Pipetting becomes inaccurate for small volumes, and impossible for smaller volumes. Also, because the high surface area to volume ratio for small volume drops, evaporation is a se ...
... which cost hundreds of thousands of dollars and are prone to mechanical problems, can only pipette down to 20 nanoliter. Pipetting becomes inaccurate for small volumes, and impossible for smaller volumes. Also, because the high surface area to volume ratio for small volume drops, evaporation is a se ...
Science 9
... Phase describes a PHYSICAL state of matter. Matter “moves” from one phase to another by physical forces such as temperature and pressure. If energy is added (e.g., increased temperature) or taken away (e.g., freezing), a physical change is created. SOLID + e = LIQUID + e = GAS + e = PLASMA Any kind ...
... Phase describes a PHYSICAL state of matter. Matter “moves” from one phase to another by physical forces such as temperature and pressure. If energy is added (e.g., increased temperature) or taken away (e.g., freezing), a physical change is created. SOLID + e = LIQUID + e = GAS + e = PLASMA Any kind ...
Period 3 Test questions GEO #3 I can identify minerals by cleavage
... •How are gold and pyrite distinguished? Pyrite streaks black, gold gold. •How is crystal form identified? The mineral grew with flat crystal faces. GEO #4 I can measure the density of minerals or rocks. •Calculate the density of this rock. (Photo of cylinder with 50 mL, photo of mass, photo of water ...
... •How are gold and pyrite distinguished? Pyrite streaks black, gold gold. •How is crystal form identified? The mineral grew with flat crystal faces. GEO #4 I can measure the density of minerals or rocks. •Calculate the density of this rock. (Photo of cylinder with 50 mL, photo of mass, photo of water ...
Properties of Minerals
... ‘Crystal habit’ describes the way that well-formed crystals grow naturally. For example, quartz is said to have a hexagonal habit, and pyrite has a cubic habit. The ‘cleavage’ of a mineral refers to how it breaks. It is related to the crystal structure of the mineral. If a mineral breaks in a regula ...
... ‘Crystal habit’ describes the way that well-formed crystals grow naturally. For example, quartz is said to have a hexagonal habit, and pyrite has a cubic habit. The ‘cleavage’ of a mineral refers to how it breaks. It is related to the crystal structure of the mineral. If a mineral breaks in a regula ...
Chapters 14
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
001 Mineral Name and Class: Quartz, Tectosilicate Chemical
... Age: OrdovicianDevonian, 490360 Mya Collection Location: Dedham Quarry Dedham, Maine 44°43’25”N, 68°36’37”W Description: Microcrystalline texture with wellsorted sand/grain. This nonfoliated rock is dull dark and hard with sizeable bands of quartz veins. It can be described as t ...
... Age: OrdovicianDevonian, 490360 Mya Collection Location: Dedham Quarry Dedham, Maine 44°43’25”N, 68°36’37”W Description: Microcrystalline texture with wellsorted sand/grain. This nonfoliated rock is dull dark and hard with sizeable bands of quartz veins. It can be described as t ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.