Dec. 15 , 2012, 9:00 am – noon - Dr. K. Brown
... 42) Ethanol (C2H5OH) has a heat of fusion of 5.01 kJ/mol. How much heat is required to melt 137 g of ethanol. A) 1.70 x 103 kJ B) 129 kJ C) 686 kJ D) 5.01 kJ E) 14.9 kJ ...
... 42) Ethanol (C2H5OH) has a heat of fusion of 5.01 kJ/mol. How much heat is required to melt 137 g of ethanol. A) 1.70 x 103 kJ B) 129 kJ C) 686 kJ D) 5.01 kJ E) 14.9 kJ ...
Chapter Five
... Therefore, if we know the mass of a given reactant, we can easily determine the number of moles of the reactant, and from there the number of moles of product produced. Remember, you must use the mole-mole relationship to carry out a conversion; the mass alone is not sufficient! Mass-Mole Proble ...
... Therefore, if we know the mass of a given reactant, we can easily determine the number of moles of the reactant, and from there the number of moles of product produced. Remember, you must use the mole-mole relationship to carry out a conversion; the mass alone is not sufficient! Mass-Mole Proble ...
Test - Angelfire
... In a redox titration, 0. 060 mol of KMnO 4 reacts completely with a solution of Sn ( NO3 )2 . ...
... In a redox titration, 0. 060 mol of KMnO 4 reacts completely with a solution of Sn ( NO3 )2 . ...
SAT - mvhs-fuhsd.org
... losing any energy, they are so far apart from each other that they have effectively no attractive forces and their speed is directly proportional to the Kelvin temperature (KineticMolecular Theory, Ideal Gas Theory) ...
... losing any energy, they are so far apart from each other that they have effectively no attractive forces and their speed is directly proportional to the Kelvin temperature (KineticMolecular Theory, Ideal Gas Theory) ...
Single Replacement Reactions - Tri
... • Incomplete combustion occurs when there isn't enough oxygen to allow the fuel (usually a hydrocarbon) to react completely. • Carbon monoxide and pure carbon will be produced in addition to carbon dioxide and water in incomplete combustion. ...
... • Incomplete combustion occurs when there isn't enough oxygen to allow the fuel (usually a hydrocarbon) to react completely. • Carbon monoxide and pure carbon will be produced in addition to carbon dioxide and water in incomplete combustion. ...
coordination compounds - Ahlcon Public School , Mayur Vihar Ph
... Give reasons for the following :Amino acids have relatively higher melting point as compared to corresponding halo acids. Because they behave like salts rather than amines or carboxylic acids. This is due to the p/o both an acidic and a basic amino gp. in the same molecule. R-CHCOOH ...
... Give reasons for the following :Amino acids have relatively higher melting point as compared to corresponding halo acids. Because they behave like salts rather than amines or carboxylic acids. This is due to the p/o both an acidic and a basic amino gp. in the same molecule. R-CHCOOH ...
Chemistry can be defined as the study of the composition, structure
... It may seem obvious that people need to breathe oxygen to survive, but plants need this element too. Many people think plants "breathe" carbon dioxide and "exhale" oxygen. But in reality, plants also "breathe" oxygen at certain times. Without oxygen, plants could not survive. Without plants, we woul ...
... It may seem obvious that people need to breathe oxygen to survive, but plants need this element too. Many people think plants "breathe" carbon dioxide and "exhale" oxygen. But in reality, plants also "breathe" oxygen at certain times. Without oxygen, plants could not survive. Without plants, we woul ...
High H2 Adsorption in a Microporous Metal-Organic
... containing open metal sites displays an uptake nearly twice as high (24.7 mg g 1) despite only a 10 % mass loss. The lack of any hysteresis in the isotherm illustrates that this physisorption process is completely reversible, and that the presence of these open metal sites does not hamper the desorp ...
... containing open metal sites displays an uptake nearly twice as high (24.7 mg g 1) despite only a 10 % mass loss. The lack of any hysteresis in the isotherm illustrates that this physisorption process is completely reversible, and that the presence of these open metal sites does not hamper the desorp ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... 7. Which hydroxides are strong bases? Sr(OH)2, KOH, NaOH, Ba(OH)2 (a). KOH, Ba(OH)2 (b). KOH, NaOH (c). KOH, NaOH, Ba(OH)2 (d) Sr(OH)2, KOH, NaOH and Ba(OH)2 8. A neutralization reaction between an acid and a metal hydroxide produces __________. (a). water and a salt (b). hydrogen gas (c). precipita ...
... 7. Which hydroxides are strong bases? Sr(OH)2, KOH, NaOH, Ba(OH)2 (a). KOH, Ba(OH)2 (b). KOH, NaOH (c). KOH, NaOH, Ba(OH)2 (d) Sr(OH)2, KOH, NaOH and Ba(OH)2 8. A neutralization reaction between an acid and a metal hydroxide produces __________. (a). water and a salt (b). hydrogen gas (c). precipita ...
Chapter_4_Reactions_in_Aqueous_Solution
... Titrations In a titration, a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that ...
... Titrations In a titration, a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that ...
Thermochemistry
... Ultimately we are talking about potential energy and kinetic energy of the system. The energy of motion (translational, rotational, vibrational, and the motion of the electrons) combined with the potential (location of atoms, forces between the nucleus and the electrons, the forces between the elec ...
... Ultimately we are talking about potential energy and kinetic energy of the system. The energy of motion (translational, rotational, vibrational, and the motion of the electrons) combined with the potential (location of atoms, forces between the nucleus and the electrons, the forces between the elec ...
reactions taking place within cells
... Thermodynamics Study of conversion of energy between heat and other forms Thermochemistry Relationship between chemical reactions and heat changes Enthalpy H Measure of energy(heat content) Change Final value minus the initial value Enthalpy change H kJmol –1 Heat energy transferred in a reaction ...
... Thermodynamics Study of conversion of energy between heat and other forms Thermochemistry Relationship between chemical reactions and heat changes Enthalpy H Measure of energy(heat content) Change Final value minus the initial value Enthalpy change H kJmol –1 Heat energy transferred in a reaction ...
Module-2-s-and-d-elements - Львівський національний медичний
... Chemical elements are classified as metals and nonmetals. The atoms of metals are electropositive and combine readily with the electronegative atoms of the nonmetals. A group of elements called metalloids, which are intermediate in properties between the metals and the nonmetals, is sometimes consid ...
... Chemical elements are classified as metals and nonmetals. The atoms of metals are electropositive and combine readily with the electronegative atoms of the nonmetals. A group of elements called metalloids, which are intermediate in properties between the metals and the nonmetals, is sometimes consid ...
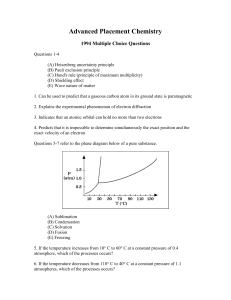
Advanced Placement Chemistry
... 38. Concentrations of colored substances are commonly measured by means of a spectrophotometer. Which of the following would ensure that correct values are obtained for the measured absorbance? I. There must be enough sample in the tube to cover the entire light path. II. The instrument must be per ...
... 38. Concentrations of colored substances are commonly measured by means of a spectrophotometer. Which of the following would ensure that correct values are obtained for the measured absorbance? I. There must be enough sample in the tube to cover the entire light path. II. The instrument must be per ...
6. Thermodynamics - Sakshi Education
... E.g.: Mass, volume, heat capacity, internal energy, enthalpy, entropy, Gibbs energy etc. The property of the system that does not depend on the total amount of the material present in the system is called an intensive property. ...
... E.g.: Mass, volume, heat capacity, internal energy, enthalpy, entropy, Gibbs energy etc. The property of the system that does not depend on the total amount of the material present in the system is called an intensive property. ...
Hard water:
... Electrochemistry: It is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor (a metal or a semiconductor) and an ionic conductor (the electrolyte), and which involve electron transfer between the electrode and the electrolyte. ...
... Electrochemistry: It is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor (a metal or a semiconductor) and an ionic conductor (the electrolyte), and which involve electron transfer between the electrode and the electrolyte. ...
AP Chapter 6 Review
... d. 2 and 3 e. 1, 2, and 3 Which one of the following statements is INCORRECT? a. The heat required to raise the temperature of 1.00 g H2O() by 1.00 C is 1.00 J. b. 1.000 calories is equal to 4.184 J. c. 1.00 J is equal to 1.00 kg·m2·s-2. d. A dietary Calorie is equal to 1000 calories. e. 1000 calo ...
... d. 2 and 3 e. 1, 2, and 3 Which one of the following statements is INCORRECT? a. The heat required to raise the temperature of 1.00 g H2O() by 1.00 C is 1.00 J. b. 1.000 calories is equal to 4.184 J. c. 1.00 J is equal to 1.00 kg·m2·s-2. d. A dietary Calorie is equal to 1000 calories. e. 1000 calo ...
Document
... 1. The measured potential of a voltaic cell is affected by changed in concentration of the reactants as the reaction proceeds and by energy losses due to heating of the cell and external circuit. 2. In order to compare the output of different cells, the standard cell potential (Eocell) is obtained a ...
... 1. The measured potential of a voltaic cell is affected by changed in concentration of the reactants as the reaction proceeds and by energy losses due to heating of the cell and external circuit. 2. In order to compare the output of different cells, the standard cell potential (Eocell) is obtained a ...
do reactions of hydroxyl radicals with metal ion go via outer sphere
... of the reacting species in solution are not known accurately, this correction was ignored for the correlation plot. Possible explanations for the behaviour displayed on the graph can be given from a consideration of theoretical and literature data. Considering first the ions which show no correlatio ...
... of the reacting species in solution are not known accurately, this correction was ignored for the correlation plot. Possible explanations for the behaviour displayed on the graph can be given from a consideration of theoretical and literature data. Considering first the ions which show no correlatio ...
AP 3rd 9 weeks notes
... 1. The measured potential of a voltaic cell is affected by changed in concentration of the reactants as the reaction proceeds and by energy losses due to heating of the cell and external circuit. 2. In order to compare the output of different cells, the standard cell potential (Eocell) is obtained a ...
... 1. The measured potential of a voltaic cell is affected by changed in concentration of the reactants as the reaction proceeds and by energy losses due to heating of the cell and external circuit. 2. In order to compare the output of different cells, the standard cell potential (Eocell) is obtained a ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.