PS_CHEM7_ch4 - WordPress.com
... • d) Ethylene glycol (HOCH2CH2OH) molecules contain polar O–H bonds, similar to water, so it would be expected to be soluble. ...
... • d) Ethylene glycol (HOCH2CH2OH) molecules contain polar O–H bonds, similar to water, so it would be expected to be soluble. ...
2 - C7Chemistry
... tetrachloromethane and hydrogen chloride gas will be produced. How many L of methane will react with 0.800 L of chlorine gas at STP? 1 L CH 4 0.800 L Cl2 0.200 L Cl2 4 L Cl2 ...
... tetrachloromethane and hydrogen chloride gas will be produced. How many L of methane will react with 0.800 L of chlorine gas at STP? 1 L CH 4 0.800 L Cl2 0.200 L Cl2 4 L Cl2 ...
chapter 5 - chemical reactions
... 3. Indicate the state of substances: (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous solution. 4. Balance the equation by introducing smallest integer (whole number) coefficients in front of each reactant and product as needed, (coefficient "1" is not shown). The chemical formula of ...
... 3. Indicate the state of substances: (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous solution. 4. Balance the equation by introducing smallest integer (whole number) coefficients in front of each reactant and product as needed, (coefficient "1" is not shown). The chemical formula of ...
Environment: The Science Behind the Stories, 4e (Withgott)
... Answer: Geothermal energy takes advantage of specific geological conditions, such as those that occur commonly along the San Andreas Fault in California, where volcanic activity near the surface heats naturally occurring water which can be captured to turn a steam turbine and generate electricity. T ...
... Answer: Geothermal energy takes advantage of specific geological conditions, such as those that occur commonly along the San Andreas Fault in California, where volcanic activity near the surface heats naturally occurring water which can be captured to turn a steam turbine and generate electricity. T ...
FREE Sample Here
... Answer: Geothermal energy takes advantage of specific geological conditions, such as those that occur commonly along the San Andreas Fault in California, where volcanic activity near the surface heats naturally occurring water which can be captured to turn a steam turbine and generate electricity. T ...
... Answer: Geothermal energy takes advantage of specific geological conditions, such as those that occur commonly along the San Andreas Fault in California, where volcanic activity near the surface heats naturally occurring water which can be captured to turn a steam turbine and generate electricity. T ...
effects of concentration and solvent composition on the electrical
... The structures of liquid alcohols are much simpler than that of water. They associate much less strongly, and form polymeric H-bonded chains, rather than large cluster, which rarely contain 5 to 7 molecules for sterically hindered alcohols [14]. When alcohol and water are mixed there is a minute but ...
... The structures of liquid alcohols are much simpler than that of water. They associate much less strongly, and form polymeric H-bonded chains, rather than large cluster, which rarely contain 5 to 7 molecules for sterically hindered alcohols [14]. When alcohol and water are mixed there is a minute but ...
The Chemical Context of Life PPT
... Why are covalent bonds more prevalent among biological molecules than ionic bonds? A. Ionic bonds only occur between metals and non-metals, and therefore aren't usually present in biological systems. B. You can have double covalent bonds, but not double ionic bonds, so covalent bonds provide more va ...
... Why are covalent bonds more prevalent among biological molecules than ionic bonds? A. Ionic bonds only occur between metals and non-metals, and therefore aren't usually present in biological systems. B. You can have double covalent bonds, but not double ionic bonds, so covalent bonds provide more va ...
The Chemical Context of Life
... Why are covalent bonds more prevalent among biological molecules than ionic bonds? A. Ionic bonds only occur between metals and non-metals, and therefore aren't usually present in biological systems. B. You can have double covalent bonds, but not double ionic bonds, so covalent bonds provide more va ...
... Why are covalent bonds more prevalent among biological molecules than ionic bonds? A. Ionic bonds only occur between metals and non-metals, and therefore aren't usually present in biological systems. B. You can have double covalent bonds, but not double ionic bonds, so covalent bonds provide more va ...
Smith Reaction- HW PSI Chemistry
... D) energy in the form of heat or light is often produced 48) A double-replacement reaction takes place when aqueous Na2CO3 reacts with aqueous Sn(NO3)2. You would expect one of the products of this reaction to be _____. A) NaNO3 B) NaSn C) Sn(CO3)2 D) CNO3 49) In a combustion reaction, one of the re ...
... D) energy in the form of heat or light is often produced 48) A double-replacement reaction takes place when aqueous Na2CO3 reacts with aqueous Sn(NO3)2. You would expect one of the products of this reaction to be _____. A) NaNO3 B) NaSn C) Sn(CO3)2 D) CNO3 49) In a combustion reaction, one of the re ...
Chapter 6
... • Note that temperature is different from heat, though the two concepts are linked. Temperature is a measure of the internal energy of the system, while heat is a measure of how energy is transferred from one system (or body) to another. The greater the heat absorbed by a material, the more rapidly ...
... • Note that temperature is different from heat, though the two concepts are linked. Temperature is a measure of the internal energy of the system, while heat is a measure of how energy is transferred from one system (or body) to another. The greater the heat absorbed by a material, the more rapidly ...
Double Displacement Reactions
... forms water as a product, but, because the reactants are in aqueous solution, there is no visible sign that water molecules have formed. Neutralization Reactions Water can form when an acid and a base are combined in a process called neutralization. Water forms when hydrogen ions from the acid join ...
... forms water as a product, but, because the reactants are in aqueous solution, there is no visible sign that water molecules have formed. Neutralization Reactions Water can form when an acid and a base are combined in a process called neutralization. Water forms when hydrogen ions from the acid join ...
AP Chemistry—Chapter 15: Applications of Aqueous Equilibria
... (a) The amount of acetylsalicylic acid in a single aspirin tablet is 325 mg, yet the tablet has a mass of 2.00 g. Calculate the mass percent of acetylsalicylic acid in the tablet. (b) The elements contained in acetylsalicylic acid are hydrogen, carbon, and oxygen. The combustion of 3.000 g of the pu ...
... (a) The amount of acetylsalicylic acid in a single aspirin tablet is 325 mg, yet the tablet has a mass of 2.00 g. Calculate the mass percent of acetylsalicylic acid in the tablet. (b) The elements contained in acetylsalicylic acid are hydrogen, carbon, and oxygen. The combustion of 3.000 g of the pu ...
KEY + + - UIC Department of Chemistry
... = 1.23 g N 2 (theoretical yield) Not possible to form 1.80 g N2. Can't make more N2 than the theoretical yield. ...
... = 1.23 g N 2 (theoretical yield) Not possible to form 1.80 g N2. Can't make more N2 than the theoretical yield. ...
this PDF file
... designing suitable reaction experiments, and therefore provides a useful guideline for the selection of processing conditions. Prior to chemical reactions, it is essential to determine the feasibility of the chemical reactions, and the nature and amount of the solid and gaseous species present in th ...
... designing suitable reaction experiments, and therefore provides a useful guideline for the selection of processing conditions. Prior to chemical reactions, it is essential to determine the feasibility of the chemical reactions, and the nature and amount of the solid and gaseous species present in th ...
Hein and Arena
... energy is used to bring about a chemical source is used to produce a chemical change. change. ...
... energy is used to bring about a chemical source is used to produce a chemical change. change. ...
Fundamentals of Chemistry
... with He and Ne) or contains eight electrons (as with Ne, Ar, Kr, Xe, Rn) is called the inert gas configuration. • Hydrogen has features that are different from any other element, it can gain or loose an electron. If it looses an electron what is left is a bare nucleus. • The hydrogen ion is very sma ...
... with He and Ne) or contains eight electrons (as with Ne, Ar, Kr, Xe, Rn) is called the inert gas configuration. • Hydrogen has features that are different from any other element, it can gain or loose an electron. If it looses an electron what is left is a bare nucleus. • The hydrogen ion is very sma ...
periodic table - Mesa Community College
... Ion: an atom that has gained or lost electrons to acquire a negative or positive charge Cation: an atom that has lost electrons and thus has a positive charge Anion: an atom that has gained electrons and thus has a negative charge Ionic compound: a neutral compound resulting from combining cations a ...
... Ion: an atom that has gained or lost electrons to acquire a negative or positive charge Cation: an atom that has lost electrons and thus has a positive charge Anion: an atom that has gained electrons and thus has a negative charge Ionic compound: a neutral compound resulting from combining cations a ...
Questionsheet 1
... The gas produced can be identified using limewater. Name the gas and the result of this test. Name of gas ............................................................................................................................................... Result of test ................................... ...
... The gas produced can be identified using limewater. Name the gas and the result of this test. Name of gas ............................................................................................................................................... Result of test ................................... ...
General Chemistry Unit 11
... In a synthesis reaction two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product is another way to identify a synthesis reaction. For example, simple hydrogen gas combined with simple oxygen gas can produce a more complex substance----water! ...
... In a synthesis reaction two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product is another way to identify a synthesis reaction. For example, simple hydrogen gas combined with simple oxygen gas can produce a more complex substance----water! ...
precipitation rxn_level_packet
... Which of the following reactions is (are) oxidation-reduction reactions? a) Zn(OH)2(s) + H2SO4(aq) ZnSO4(aq) + 2 H2O(l) b) Ca(s) + 2 H2O(l) Ca(OH)2(s) + H2(g) ...
... Which of the following reactions is (are) oxidation-reduction reactions? a) Zn(OH)2(s) + H2SO4(aq) ZnSO4(aq) + 2 H2O(l) b) Ca(s) + 2 H2O(l) Ca(OH)2(s) + H2(g) ...
1994 Released Exam
... Use your time effectively, workingasrapidlyas you canwithoutlosingaccuracy.Do not spendtoo muchtimeon questionsthatare too difficult. Go on to otherquestions andcomebackto the difficult oneslaterif you havetime. It is not expectedthateveryonewill be ableto answerall the multiple-choicequestions. ...
... Use your time effectively, workingasrapidlyas you canwithoutlosingaccuracy.Do not spendtoo muchtimeon questionsthatare too difficult. Go on to otherquestions andcomebackto the difficult oneslaterif you havetime. It is not expectedthateveryonewill be ableto answerall the multiple-choicequestions. ...
2006 Practice Final Exam - Department of Chemistry | Oregon State
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your test form number (listed above), l ...
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your test form number (listed above), l ...
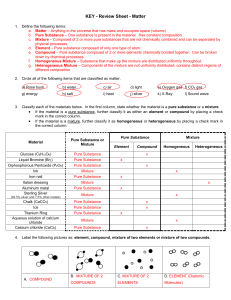
Matter Test Review Sheet
... f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Heterogeneous Mixture – Components of the mixture are not uniformly distributed, contains distinct regions of different composition. 2. Circle all of the following items that are classified as matter ...
... f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Heterogeneous Mixture – Components of the mixture are not uniformly distributed, contains distinct regions of different composition. 2. Circle all of the following items that are classified as matter ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.