Topic 1: Quantitative Chemistry
... 5.1 Exothermic and endothermic reactions 5.1.1 Define the terms exothermic reaction, endothermic reaction, and standard enthalpy change of a reaction (∆HӨ) 5.1.2 State that combustion and neutralization are exothermic reactions. 5.1.3 Apply the relationship between temperature change, enthalpy chang ...
... 5.1 Exothermic and endothermic reactions 5.1.1 Define the terms exothermic reaction, endothermic reaction, and standard enthalpy change of a reaction (∆HӨ) 5.1.2 State that combustion and neutralization are exothermic reactions. 5.1.3 Apply the relationship between temperature change, enthalpy chang ...
National 5 - Deans Community High School
... Copy the graph showing reaction A and add the corresponding curves which could have been obtained for experiments B, C and D. (Label each curve clearly.) 18. The collision theory states that for two molecules to react, they must first collide with one another. Use the collision theory to explain the ...
... Copy the graph showing reaction A and add the corresponding curves which could have been obtained for experiments B, C and D. (Label each curve clearly.) 18. The collision theory states that for two molecules to react, they must first collide with one another. Use the collision theory to explain the ...
Chemistry notes Important terms *Mass of element in a sample
... kinetics and equilibrium are distinct aspects of a chemical reaction, thus the rate extent of a reaction are related. When the forward and reverse reactions occur at the same rate, the system has reached dynamic equilibrium. The equilibrium constant (K) is a number based on a particular ratio ...
... kinetics and equilibrium are distinct aspects of a chemical reaction, thus the rate extent of a reaction are related. When the forward and reverse reactions occur at the same rate, the system has reached dynamic equilibrium. The equilibrium constant (K) is a number based on a particular ratio ...
practice problems
... Plan: We will use Hess’s law. In doing so, we first note the numbers of moles of substances among the reactants and products in the target equation, (3). We then manipulate equations (1) and (2) to give the same number of moles of these substances, so that when the resulting equations are added, we ...
... Plan: We will use Hess’s law. In doing so, we first note the numbers of moles of substances among the reactants and products in the target equation, (3). We then manipulate equations (1) and (2) to give the same number of moles of these substances, so that when the resulting equations are added, we ...
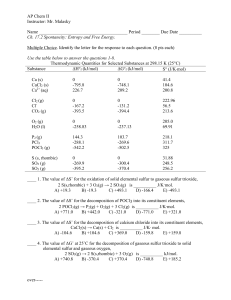
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... Free Response. (36 pts) Acetylene gas, C2H2, is used in gas welding procedures and is a very important commercial gas. Use the data below to answer the following questions about the combustion of acetylene gas. Assume all reactions occur at 25◦C. Substance ∆Hf◦ (kJ mol-1) S◦ (J mol-1 K-1) ...
... Free Response. (36 pts) Acetylene gas, C2H2, is used in gas welding procedures and is a very important commercial gas. Use the data below to answer the following questions about the combustion of acetylene gas. Assume all reactions occur at 25◦C. Substance ∆Hf◦ (kJ mol-1) S◦ (J mol-1 K-1) ...
Honors Chemistry
... If we increase the amount of A or B, the system shifts to the If we increase the amount of C or D, the system shifts to the If we decrease the amount of C or D, the system shifts to the (a good way to increase ...
... If we increase the amount of A or B, the system shifts to the If we increase the amount of C or D, the system shifts to the If we decrease the amount of C or D, the system shifts to the (a good way to increase ...
Chapter6 - GEOCITIES.ws
... A given quantity of heat will raise the temperature of a gas more if the sample is small. Reason: The Absolute Temperature is directly proportional to the Average Kinetic Energy (Ek). - When you add heat to a gas, you increase its total Ek - The increase in Ek will be distributed over all the molecu ...
... A given quantity of heat will raise the temperature of a gas more if the sample is small. Reason: The Absolute Temperature is directly proportional to the Average Kinetic Energy (Ek). - When you add heat to a gas, you increase its total Ek - The increase in Ek will be distributed over all the molecu ...
Chapter 4: Solution Chemistry and the Hydrosphere
... Oxidation Number (or Oxidation State): actual or hypothetical charge of an atom in a compound if it existed as a monatomic ion ...
... Oxidation Number (or Oxidation State): actual or hypothetical charge of an atom in a compound if it existed as a monatomic ion ...
Solution
... A 0.5662-g sample of an ionic compound containing chloride ions and an unknown metal is dissolved in water and treated with an excess of AgNO3. If 1.0882 g of AgCl precipitate forms, what is the percent by mass of Cl in the original compound? ...
... A 0.5662-g sample of an ionic compound containing chloride ions and an unknown metal is dissolved in water and treated with an excess of AgNO3. If 1.0882 g of AgCl precipitate forms, what is the percent by mass of Cl in the original compound? ...
Subject Materials for Chemistry
... Glycerol decreases the rate of reaction. So glycerol is –ve catalyst. No, Catalyst doesn’t undergo any change chemically. A Catalyst may be recovered in mass and composition at the end of the chemical reaction. 6. What is the effect of temperature on the following? i) Dissociation of an electrolyte ...
... Glycerol decreases the rate of reaction. So glycerol is –ve catalyst. No, Catalyst doesn’t undergo any change chemically. A Catalyst may be recovered in mass and composition at the end of the chemical reaction. 6. What is the effect of temperature on the following? i) Dissociation of an electrolyte ...
Document
... The pH of a given solution is unknown. Indicators 1 and 3 turn yellow in this solution. Between which values will the pH of this solution fall? A) Between 0 and 5 C) Between 5 and 12 B) Between 5 and 6 D) Between 6 and 12 16. Answer the questions below using the 3 indicators pH 1 ...
... The pH of a given solution is unknown. Indicators 1 and 3 turn yellow in this solution. Between which values will the pH of this solution fall? A) Between 0 and 5 C) Between 5 and 12 B) Between 5 and 6 D) Between 6 and 12 16. Answer the questions below using the 3 indicators pH 1 ...
What is Thermodynamics?
... measure of how much energy is dispersed, per unit temperature, in any process. This energy is not available to do work. In a reversible process ...
... measure of how much energy is dispersed, per unit temperature, in any process. This energy is not available to do work. In a reversible process ...
Chapter 5 Chemical Equilibrium 1 State whether each of the
... (c) You should have found that Hvap is smaller at the higher temperature. Why is this so? This is because at higher temperature, the water molecules already have higher energy, so less is required to vaporize them from the liquid into the gas phase. ...
... (c) You should have found that Hvap is smaller at the higher temperature. Why is this so? This is because at higher temperature, the water molecules already have higher energy, so less is required to vaporize them from the liquid into the gas phase. ...
Macrocyclic Leaflets
... three phosphonate anions, 1NH, five nitrate anions, and eight water molecules. Interestingly, 3 also forms a leaflet structure like 2, but has a complicated polymeric coordination network as the backbone, in which Cd(II) cations adopt octahedral geometry and form two unique Cd‚‚‚Cd metal contacts (3 ...
... three phosphonate anions, 1NH, five nitrate anions, and eight water molecules. Interestingly, 3 also forms a leaflet structure like 2, but has a complicated polymeric coordination network as the backbone, in which Cd(II) cations adopt octahedral geometry and form two unique Cd‚‚‚Cd metal contacts (3 ...
First Law of Thermodynamics
... in Kelvin. However, since the Celsius and Kelvin scales differ only by an additive constant, temperature changes are the same in the two systems of units. Note that the heat capacity of the potassium nitrate is not required in this problem. A foxy instructor might provide the number on an exam as a ...
... in Kelvin. However, since the Celsius and Kelvin scales differ only by an additive constant, temperature changes are the same in the two systems of units. Note that the heat capacity of the potassium nitrate is not required in this problem. A foxy instructor might provide the number on an exam as a ...
Qualitative Analysis Lab
... This lab will be divided into two parts, each part being performed during a different lab period. During the first lab period, you will perform each of the tests described in the introduction and note the observations for yourself. You will also practice writing the correct, balanced molecular and n ...
... This lab will be divided into two parts, each part being performed during a different lab period. During the first lab period, you will perform each of the tests described in the introduction and note the observations for yourself. You will also practice writing the correct, balanced molecular and n ...
AP Chemistry Review Preparing for the AP
... Focus on your weakest areas; it is doubtful you can do/know everything. The AP Chemistry Exam is designed so that it is impossible to know absolutely everything on it (in case you haven’t noticed). Review your incorrect MC from the Practice Exam and understand the concepts. Know the 6 strong acids H ...
... Focus on your weakest areas; it is doubtful you can do/know everything. The AP Chemistry Exam is designed so that it is impossible to know absolutely everything on it (in case you haven’t noticed). Review your incorrect MC from the Practice Exam and understand the concepts. Know the 6 strong acids H ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.