Lecture 1 Atomic Structure
... Discharge tube experiments provided strong evidence for the existence of subatomic particles. A discharge tube is a glass tube having two electrodes sealed in at each end. It is connected to a high voltage battery to provide required voltage and to a vacuum pump to evacuate air or gas from the tube. ...
... Discharge tube experiments provided strong evidence for the existence of subatomic particles. A discharge tube is a glass tube having two electrodes sealed in at each end. It is connected to a high voltage battery to provide required voltage and to a vacuum pump to evacuate air or gas from the tube. ...
Sample Paper Chemistry - Educomp Solutions Ltd.
... (b) An optically active compound having molecular formula C7H15Br reacts with aqueous KOH to give a racemic mixture of products. Write the mechanism involved in this reaction. ...
... (b) An optically active compound having molecular formula C7H15Br reacts with aqueous KOH to give a racemic mixture of products. Write the mechanism involved in this reaction. ...
Question 2 - The King`s School, Canterbury
... 1. (a) A student investigated the effect of light intensity on leaf size. The student collected 25 leaves from bramble plants at two different sites. One of the sites was a woodland with low light levels and the other, a woodland with high light levels. The student found the average surface area of ...
... 1. (a) A student investigated the effect of light intensity on leaf size. The student collected 25 leaves from bramble plants at two different sites. One of the sites was a woodland with low light levels and the other, a woodland with high light levels. The student found the average surface area of ...
Chapter 9 Stoichiometry
... Enthalpy is the amount of heat that a substance has at a given temperature and pressure (see Table 8.1 pg 190) The heat of a reaction is the heat that is released or absorbed during a chemical reaction. Heat of Reaction is represented by The symbol H ...
... Enthalpy is the amount of heat that a substance has at a given temperature and pressure (see Table 8.1 pg 190) The heat of a reaction is the heat that is released or absorbed during a chemical reaction. Heat of Reaction is represented by The symbol H ...
homework_#1_10
... of atoms on each side and the same total mass on each side. You DO NOT have the same number of MOLES on each side (7 on the left, 6 on the right) or VOLUME (7 x 22.4 Liters on the left, 6 x 22.4 on the right) or MOLECULES (7 on the left, 6 on the right) ...
... of atoms on each side and the same total mass on each side. You DO NOT have the same number of MOLES on each side (7 on the left, 6 on the right) or VOLUME (7 x 22.4 Liters on the left, 6 x 22.4 on the right) or MOLECULES (7 on the left, 6 on the right) ...
Chapter 6
... H2O. (although incomplete burning does cause some by-products like carbon monoxide) • Combustion is a type of oxidation-reduction reactions. ...
... H2O. (although incomplete burning does cause some by-products like carbon monoxide) • Combustion is a type of oxidation-reduction reactions. ...
Week 1 - University of Guelph
... – the equations represent the amounts in moles. In a), we move from 1.00 mole of gaseous CO2 on the reactant side to 0 moles of gas on the product side. Work is done ___ the system ____ the surroundings. This is an example of a __________________. Work done is then ____________ (+/–). First determin ...
... – the equations represent the amounts in moles. In a), we move from 1.00 mole of gaseous CO2 on the reactant side to 0 moles of gas on the product side. Work is done ___ the system ____ the surroundings. This is an example of a __________________. Work done is then ____________ (+/–). First determin ...
Are You Ready For S201
... in which are located the _____________ that carry most of the genetic information of the organism. These structures consist of a long double-stranded molecule called _______________________ (abbreviated to____) and various _____________ molecules. In _______________ organisms (such as bacteria) the ...
... in which are located the _____________ that carry most of the genetic information of the organism. These structures consist of a long double-stranded molecule called _______________________ (abbreviated to____) and various _____________ molecules. In _______________ organisms (such as bacteria) the ...
Review for Physical Science Test #2
... To tell a strong acid from a weak acid To tell an acid from a neutral solution To tell a strong base from a weak base To create a temporary tattoo on your little sister’s face right before picture day. ...
... To tell a strong acid from a weak acid To tell an acid from a neutral solution To tell a strong base from a weak base To create a temporary tattoo on your little sister’s face right before picture day. ...
Supported-Metal Catalysts
... reduced to form cations with lower valences. Spectroscopic measurements indicate that an electron is transferred from the cation (such as Ti3+ or Nb4+) to the metal particle. This, in turn, leads to profound changes in the catalytic and chemisorption properties and the morphology of the metal partic ...
... reduced to form cations with lower valences. Spectroscopic measurements indicate that an electron is transferred from the cation (such as Ti3+ or Nb4+) to the metal particle. This, in turn, leads to profound changes in the catalytic and chemisorption properties and the morphology of the metal partic ...
Test review
... _____ 3. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. The total pressure is measured to be 9.0 atm. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? (A) It depends ...
... _____ 3. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. The total pressure is measured to be 9.0 atm. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? (A) It depends ...
ConcepTest On Simple Redox Reactions
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
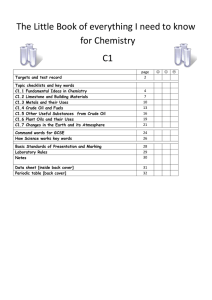
The Big book of C1 chemistry
... Unreactive metals such as gold are found in the Earth as the metal itself but most metals are found as compounds that require chemical reactions to extract the metal. Metals that are less reactive than carbon can be extracted from their oxides by reduction with carbon, for example iron oxide is redu ...
... Unreactive metals such as gold are found in the Earth as the metal itself but most metals are found as compounds that require chemical reactions to extract the metal. Metals that are less reactive than carbon can be extracted from their oxides by reduction with carbon, for example iron oxide is redu ...
in a Chemical Reactor - Max-Planck
... in a conventional reactor. An example of this would be in the case where two substances are to chemically interact only up to a certain point, whereas they would normally overstep this boundary. Oxygen is a substance that has a hard time controlling its chemical ...
... in a conventional reactor. An example of this would be in the case where two substances are to chemically interact only up to a certain point, whereas they would normally overstep this boundary. Oxygen is a substance that has a hard time controlling its chemical ...
AP® Chemistry 2009 Free-Response Questions - AP Central
... NO CALCULATORS MAY BE USED FOR PART B. Answer Question 4 below. The Section II score weighting for this question is 10 percent. 4. For each of the following three reactions, write a balanced equation in part (i) and answer the question in part (ii). In part (i), coefficients should be in terms of lo ...
... NO CALCULATORS MAY BE USED FOR PART B. Answer Question 4 below. The Section II score weighting for this question is 10 percent. 4. For each of the following three reactions, write a balanced equation in part (i) and answer the question in part (ii). In part (i), coefficients should be in terms of lo ...
Balancing Redox Equations
... Oxidation Number - The charge that an atom would have if the compound in which it were found were ionic. The rules: 1) The sum of the oxidation numbers of the atoms in a molecule must be equal to the overall charge on the molecule. 2) To assign a number to a transition metal ion (not listed in the t ...
... Oxidation Number - The charge that an atom would have if the compound in which it were found were ionic. The rules: 1) The sum of the oxidation numbers of the atoms in a molecule must be equal to the overall charge on the molecule. 2) To assign a number to a transition metal ion (not listed in the t ...
physical setting chemistry
... Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. All answers in your answer booklet should be written in pen, except for graphs and drawings, which should be done in pencil. You may use scrap paper to work out the answers to the ...
... Part B–2 and Part C in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. All answers in your answer booklet should be written in pen, except for graphs and drawings, which should be done in pencil. You may use scrap paper to work out the answers to the ...
Slajd 1
... forth between states along the same path. When 1 mol of water is frozen at 1 atm at 0 °C to form 1 mol of ice, q = ∆Hvap of heat is removed. To reverse the process, q = ∆Hvap must be added to the 1 mol of ice at 0°C and 1 atm to form 1 mol of water at 0 °C ...
... forth between states along the same path. When 1 mol of water is frozen at 1 atm at 0 °C to form 1 mol of ice, q = ∆Hvap of heat is removed. To reverse the process, q = ∆Hvap must be added to the 1 mol of ice at 0°C and 1 atm to form 1 mol of water at 0 °C ...
CH100: Fundamentals for Chemistry
... Metals have low specific heat capacity Non-metals have higher specific heat capacity Water has an unusually large specific heat capacity ...
... Metals have low specific heat capacity Non-metals have higher specific heat capacity Water has an unusually large specific heat capacity ...
spring semester review
... a) HNO2 + K+ + OH- ---> KNO2 + H2O b) HNO2 + H2O ----> NO2- + H3O+ c) HNO2 + KOH ----> K+ + NO2- + H2O d) HNO2 + OH- ----> NO2- + H2O e) H+ + OH- ----> H2O 34. A solution of ammonia is titrated with hydrochloric acid. At the equivalence point, phenolphthalein will be what color? a) colorless b) pink ...
... a) HNO2 + K+ + OH- ---> KNO2 + H2O b) HNO2 + H2O ----> NO2- + H3O+ c) HNO2 + KOH ----> K+ + NO2- + H2O d) HNO2 + OH- ----> NO2- + H2O e) H+ + OH- ----> H2O 34. A solution of ammonia is titrated with hydrochloric acid. At the equivalence point, phenolphthalein will be what color? a) colorless b) pink ...
acids - WordPress.com
... • In this method, both the reactants used are in the aqueous state thus excess reagent cannot be easily removed from the product which is in the same state. Thus exact quantities of reactants must be used to ensure that product is not contaminated by excess reagent. • The two main steps involved ar ...
... • In this method, both the reactants used are in the aqueous state thus excess reagent cannot be easily removed from the product which is in the same state. Thus exact quantities of reactants must be used to ensure that product is not contaminated by excess reagent. • The two main steps involved ar ...
Topic 1: Quantitative Chemistry
... 5.1 Exothermic and endothermic reactions 5.1.1 Define the terms exothermic reaction, endothermic reaction, and standard enthalpy change of a reaction (∆HӨ) 5.1.2 State that combustion and neutralization are exothermic reactions. 5.1.3 Apply the relationship between temperature change, enthalpy chang ...
... 5.1 Exothermic and endothermic reactions 5.1.1 Define the terms exothermic reaction, endothermic reaction, and standard enthalpy change of a reaction (∆HӨ) 5.1.2 State that combustion and neutralization are exothermic reactions. 5.1.3 Apply the relationship between temperature change, enthalpy chang ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.