Atomic Structure Practice Test
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
Mechanistic studies on the diazo transfer reaction
... presented in this Letter, would help one prepare specifically labeled proteins/reactants which might be useful for IR spectroscopic tagging of amines. We first confirmed the diazo transfer as opposed to the SNi path which would also result in retention of configuration. To the best of our knowledge, it ...
... presented in this Letter, would help one prepare specifically labeled proteins/reactants which might be useful for IR spectroscopic tagging of amines. We first confirmed the diazo transfer as opposed to the SNi path which would also result in retention of configuration. To the best of our knowledge, it ...
Class Notes
... The law of conservation of mass (aka: the law of conservation of matter) says that during a chemical reaction or a physical change mass is conserved; mass is neither created nor destroyed. This implies that the atoms that were there in the reactants (before the chemical change) must be there in the ...
... The law of conservation of mass (aka: the law of conservation of matter) says that during a chemical reaction or a physical change mass is conserved; mass is neither created nor destroyed. This implies that the atoms that were there in the reactants (before the chemical change) must be there in the ...
6.02 × 1023 molecules = 1 mole
... One of the most well-known numbers in the study of chemistry is number of units in a mole. The number of units in a mole is called Avogadro’s number (named after the Italian physicist). The mole is defined as the number of atoms in 12.0 grams of 12C. As you can tell from the equality below, the mole ...
... One of the most well-known numbers in the study of chemistry is number of units in a mole. The number of units in a mole is called Avogadro’s number (named after the Italian physicist). The mole is defined as the number of atoms in 12.0 grams of 12C. As you can tell from the equality below, the mole ...
Fractionation of the isotopes of carbon and hydrogen in biosynthetic
... An arbitrary sequence of reactions is indicated schematically in Figure 2. Analysis of this system will provide a useful introduction to studies of real organisms. Reactant A could, for example, represent a particular carbon position in a two- or three-carbon product of metabolism or photosynthesis. ...
... An arbitrary sequence of reactions is indicated schematically in Figure 2. Analysis of this system will provide a useful introduction to studies of real organisms. Reactant A could, for example, represent a particular carbon position in a two- or three-carbon product of metabolism or photosynthesis. ...
actiona actionation of FFFFFrrrrractiona
... An arbitrary sequence of reactions is indicated schematically in Figure 2. Analysis of this system will provide a useful introduction to studies of real organisms. Reactant A could, for example, represent a particular carbon position in a two- or three-carbon product of metabolism or photosynthesis. ...
... An arbitrary sequence of reactions is indicated schematically in Figure 2. Analysis of this system will provide a useful introduction to studies of real organisms. Reactant A could, for example, represent a particular carbon position in a two- or three-carbon product of metabolism or photosynthesis. ...
Prescott`s Microbiology, 9th Edition 12 Anabolism: The Use of
... 3. The reduction phase—3-phosphoglycerate is reduced to glyceraldehyde 3-phosphate 4. The regeneration phase—a series of reactions is used to regenerate ribulose 1,5-bisphosphate and to produce carbohydrates such as fructose and glucose; this phase is similar to the pentose phosphate pathway and inv ...
... 3. The reduction phase—3-phosphoglycerate is reduced to glyceraldehyde 3-phosphate 4. The regeneration phase—a series of reactions is used to regenerate ribulose 1,5-bisphosphate and to produce carbohydrates such as fructose and glucose; this phase is similar to the pentose phosphate pathway and inv ...
Chapter 3—Time and Geology
... half-life (39): The time in which one-half of an original amount of a radioactive atoms decays to daughter products. Holocene Series (34): A term sometimes used to designate the period of time since the last major episode of glaciation. The term is equivalent to Holocene and ranges from 10,000-12,0 ...
... half-life (39): The time in which one-half of an original amount of a radioactive atoms decays to daughter products. Holocene Series (34): A term sometimes used to designate the period of time since the last major episode of glaciation. The term is equivalent to Holocene and ranges from 10,000-12,0 ...
Chem101 - Lecture 2 Elements Elements
... determined by comparing them to the mass of the carbon-12 isotope. • The unit of mass that is used is called the atomic mass unit and is represented by the symbol u. • The atomic mass unit is equal to exactly 1/12 the mass of the carbon-12 ...
... determined by comparing them to the mass of the carbon-12 isotope. • The unit of mass that is used is called the atomic mass unit and is represented by the symbol u. • The atomic mass unit is equal to exactly 1/12 the mass of the carbon-12 ...
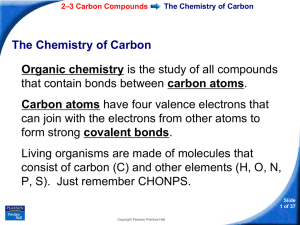
2–3 Carbon Compounds
... Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Carbon atoms have four valence electrons that can join with the electrons from other atoms to form strong covalent bonds. Living organisms are made of molecules that consist of carbon (C) and other elements (H, ...
... Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Carbon atoms have four valence electrons that can join with the electrons from other atoms to form strong covalent bonds. Living organisms are made of molecules that consist of carbon (C) and other elements (H, ...
Topic 2.1 Atomic Structure Notes Topic 2.1 Atomic
... masses and abundance of isotopes from given data. Three isotopes of magnesium occur in nature. Their abundances and masses, determined by mass spectrometry, are listed in the table on the right. Use this information to calculate the atomic weight of magnesium. ...
... masses and abundance of isotopes from given data. Three isotopes of magnesium occur in nature. Their abundances and masses, determined by mass spectrometry, are listed in the table on the right. Use this information to calculate the atomic weight of magnesium. ...
radioactivity - the Scientia Review
... There are particles smaller than protons, neutrons, and electrons. Protons and neutrons are made up of particles called quarks. There are only two types of quarks (up and down), and different combinations of these make up protons and neutrons. Very little is known about quarks. They seem to have the ...
... There are particles smaller than protons, neutrons, and electrons. Protons and neutrons are made up of particles called quarks. There are only two types of quarks (up and down), and different combinations of these make up protons and neutrons. Very little is known about quarks. They seem to have the ...
The Mole
... Atoms (molecules for diatomic molecules) Molecular compounds (molecules) Ionic compounds (formula units) One mole of any substance is always said to contain 6.02x1023 representative particles ...
... Atoms (molecules for diatomic molecules) Molecular compounds (molecules) Ionic compounds (formula units) One mole of any substance is always said to contain 6.02x1023 representative particles ...
Chemical Reactions and The Mole
... The mole, historically, has frustrated many a student. I offer this narrative in hopes of keeping this frustration from occurring. The phrase “Less of them” when discussing the amount of a substance is correct, not the phrase “Less of it”. The difference is in the word “them”. That indicates a numbe ...
... The mole, historically, has frustrated many a student. I offer this narrative in hopes of keeping this frustration from occurring. The phrase “Less of them” when discussing the amount of a substance is correct, not the phrase “Less of it”. The difference is in the word “them”. That indicates a numbe ...
Chemical Formulas and Chemical Compounds
... a. How many elements make up this compound? b. How many oxygen atoms are present in one molecule of C7H5(NO2)3? c. How many atoms in total are present in one molecule of C7H5(NO2)3? d. How many atoms are present in a sample of 2.0 ⫻ 1023 molecules of C7H5(NO2)3? ...
... a. How many elements make up this compound? b. How many oxygen atoms are present in one molecule of C7H5(NO2)3? c. How many atoms in total are present in one molecule of C7H5(NO2)3? d. How many atoms are present in a sample of 2.0 ⫻ 1023 molecules of C7H5(NO2)3? ...
Unit 2.4 Understanding the Elements Listed on the Periodic Table
... important so that we can compare experimental data from one lab to another and make sure we all are talking about the same thing. ...
... important so that we can compare experimental data from one lab to another and make sure we all are talking about the same thing. ...
Document
... Crystalline potassium hydrogen phthalate KHC8H4O4 is used to standardize basic solutions. If 1.548 g of this salt is titrated with a solution of Ca(OH)2, the end point is reached when 42.37 mL of the solution has been added. What is the molarity of the Ca(OH)2 solution? (Atomic weights: C = 12.01, O ...
... Crystalline potassium hydrogen phthalate KHC8H4O4 is used to standardize basic solutions. If 1.548 g of this salt is titrated with a solution of Ca(OH)2, the end point is reached when 42.37 mL of the solution has been added. What is the molarity of the Ca(OH)2 solution? (Atomic weights: C = 12.01, O ...
Slajdovi sa predavanja
... frequency (RF) range, typically 40-800 MHz, to the sample. These pulses of RF cause the nuclei to rotate away from their equilibrium position and they start to precess (rotate) around the axis of the magnetic field. The exact frequency at which the nuclei precess is related to both the chemical and ...
... frequency (RF) range, typically 40-800 MHz, to the sample. These pulses of RF cause the nuclei to rotate away from their equilibrium position and they start to precess (rotate) around the axis of the magnetic field. The exact frequency at which the nuclei precess is related to both the chemical and ...
1a) Charged particles in matter :-
... Defects of Rutherford’s model of the atom :Any particle in a circular orbit would undergo acceleration and during acceleration the charged particle would radiate energy. So the revolving electrons would lose energy and fall into the nucleus and the atom would be unstable. We know that atoms are stab ...
... Defects of Rutherford’s model of the atom :Any particle in a circular orbit would undergo acceleration and during acceleration the charged particle would radiate energy. So the revolving electrons would lose energy and fall into the nucleus and the atom would be unstable. We know that atoms are stab ...
CChemical Reactions and Radioactivity
... A clear understanding of chemistry will contribute immensely to the goals of Science 10 outlined by British Columbia’s IRPs (as briefly described above), in that knowledge of basic laws, principles, and concepts in chemistry will provide students with a basis for understanding mechanism behind many ...
... A clear understanding of chemistry will contribute immensely to the goals of Science 10 outlined by British Columbia’s IRPs (as briefly described above), in that knowledge of basic laws, principles, and concepts in chemistry will provide students with a basis for understanding mechanism behind many ...
precipitation rxn_level_packet
... Directions for the following 4 reactions: a. In one well of a well-plate, add three drops of each substance. b. Write down your observations for the reactants above. c. In parenthesis provided above, indicate if the product is soluble with an “aq” or forms a precipitate (solid) with an “s.” 1. Write ...
... Directions for the following 4 reactions: a. In one well of a well-plate, add three drops of each substance. b. Write down your observations for the reactants above. c. In parenthesis provided above, indicate if the product is soluble with an “aq” or forms a precipitate (solid) with an “s.” 1. Write ...
3. What is the empirical formula of a compound that is
... As you learned in health and biology, food energy typically comes from carbohydrates, proteins and fats. The amount of energy that the body can use per gram of these substances is not the same. The following balanced exothermic reaction represents combustion (respiration) of glucose (a carbohydrate) ...
... As you learned in health and biology, food energy typically comes from carbohydrates, proteins and fats. The amount of energy that the body can use per gram of these substances is not the same. The following balanced exothermic reaction represents combustion (respiration) of glucose (a carbohydrate) ...
Final Review 2006
... ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle d. octet rule ____ 77. Multiple covalent bonds may occur in atoms that contain carbon, nitrogen, or ...
... ____ 76. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle d. octet rule ____ 77. Multiple covalent bonds may occur in atoms that contain carbon, nitrogen, or ...
2.ATOMS, MOLECULES, AND IONS
... b. You recognize the fact that whenever a cation can have multiple oxidation states (1+, 2+, and 5+ in this case) the name of the compound must indicate the charge. Therefore, the names of the compounds in part (a) would be exy(I) sulfate, exy(II) sulfate, and exy(V) sulfate, respectively. 2.26 a. T ...
... b. You recognize the fact that whenever a cation can have multiple oxidation states (1+, 2+, and 5+ in this case) the name of the compound must indicate the charge. Therefore, the names of the compounds in part (a) would be exy(I) sulfate, exy(II) sulfate, and exy(V) sulfate, respectively. 2.26 a. T ...
History and Current Status of the Plastics Industry
... – Occurs when two metal atoms are in close proximity.Both atoms have tendency to give up electrons. Electrons are free to move about entire atoms structure – Releasing electrons yields a lower energy state. – The metal atoms approach each other and give up electrons when in close proximity to a sea ...
... – Occurs when two metal atoms are in close proximity.Both atoms have tendency to give up electrons. Electrons are free to move about entire atoms structure – Releasing electrons yields a lower energy state. – The metal atoms approach each other and give up electrons when in close proximity to a sea ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.