Slide 1
... e.g. C3 vs C4. Slope could vary from positive to negative depending on direction of shift. (Fig. 1C, J.G. Wynn, et al., 2006) ...
... e.g. C3 vs C4. Slope could vary from positive to negative depending on direction of shift. (Fig. 1C, J.G. Wynn, et al., 2006) ...
Chapter 11: The rise of oxygen and ozone – ppt
... isotopes – same number of protons and electrons, different number of neutrons. Affects the atomic weight, but not the electrons, and therefore not the types of chemical reactions it undergoes. Oxidation states – associated with the number of electrons either given or taken in a chemical bond. Oxygen ...
... isotopes – same number of protons and electrons, different number of neutrons. Affects the atomic weight, but not the electrons, and therefore not the types of chemical reactions it undergoes. Oxidation states – associated with the number of electrons either given or taken in a chemical bond. Oxygen ...
Aalseth-icpms - Berkeley Cosmology Group
... – Hard to measure at necessary levels – May have to depend on higher-level validation of equilibrium behavior for a particular system, then process knowledge ...
... – Hard to measure at necessary levels – May have to depend on higher-level validation of equilibrium behavior for a particular system, then process knowledge ...
turcuman s - Revista de Chimie
... system due to the crystallographic characteristics of the ligand that keeps its monoclinic crystalline structure. The elemental cell parameters are shown in table 6. Based on the elemental cell parameters of FeL3 and CoL2 x 2H2O compounds, it results that in both cases the values of a and c paramete ...
... system due to the crystallographic characteristics of the ligand that keeps its monoclinic crystalline structure. The elemental cell parameters are shown in table 6. Based on the elemental cell parameters of FeL3 and CoL2 x 2H2O compounds, it results that in both cases the values of a and c paramete ...
Atomic Mass Review Sheet
... The mass number is equal to the sum of the protons and neutrons in an atom. Remember it this way, the mass number is equal to the total number of protons plus neutrons. The mass number can be used to estimate atomic mass (see atomic mass below) The mass number is often indicated as a superscript in ...
... The mass number is equal to the sum of the protons and neutrons in an atom. Remember it this way, the mass number is equal to the total number of protons plus neutrons. The mass number can be used to estimate atomic mass (see atomic mass below) The mass number is often indicated as a superscript in ...
Muhammad Danish - Chemistry Department
... class of organotin compounds as they show different sets of 1H and 13C-NMR signals for Rgroups attached to Sn(IV) and only one set of signals for carboxylate ligand. A similar dichotomy is observed 119Sn-NMR spectra1,2. Two types of signals are only possible if there are two environments for the R-g ...
... class of organotin compounds as they show different sets of 1H and 13C-NMR signals for Rgroups attached to Sn(IV) and only one set of signals for carboxylate ligand. A similar dichotomy is observed 119Sn-NMR spectra1,2. Two types of signals are only possible if there are two environments for the R-g ...
Carbon-Based Molecules
... chemical energy in organisms. Animal fats are found in foods such as meat and butter. You know plant fats as oils, such as olive oil and peanut oil. The structures of fats and oils are similar. They both consist of a molecule called glycerol (glihs-uh-rawl) bonded to molecules called fatty acids. Fa ...
... chemical energy in organisms. Animal fats are found in foods such as meat and butter. You know plant fats as oils, such as olive oil and peanut oil. The structures of fats and oils are similar. They both consist of a molecule called glycerol (glihs-uh-rawl) bonded to molecules called fatty acids. Fa ...
File
... Many lipids are formed from glycerol and a. fatty acids. b. monosaccharides. c. amino acids. ...
... Many lipids are formed from glycerol and a. fatty acids. b. monosaccharides. c. amino acids. ...
pages 46-50
... chemical energy in organisms. Animal fats are found in foods such as meat and butter. You know plant fats as oils, such as olive oil and peanut oil. The structures of fats and oils are similar. They both consist of a molecule called glycerol (glihs-uh-rawl) bonded to molecules called fatty acids. Fa ...
... chemical energy in organisms. Animal fats are found in foods such as meat and butter. You know plant fats as oils, such as olive oil and peanut oil. The structures of fats and oils are similar. They both consist of a molecule called glycerol (glihs-uh-rawl) bonded to molecules called fatty acids. Fa ...
Chapter 3 – part I Sections 1-3
... • What is oxidized and reduced are always reactants, the products are the result of the redox. • So if asked “what is ox or red?”, answer is reactant ...
... • What is oxidized and reduced are always reactants, the products are the result of the redox. • So if asked “what is ox or red?”, answer is reactant ...
Lecture 5 – Chemical Reactions
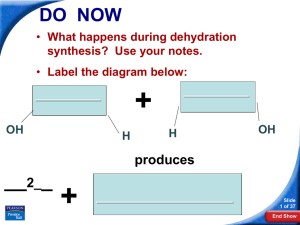
... They can be recognized because they have only one reactant, which breaks down to produce two or more products. ...
... They can be recognized because they have only one reactant, which breaks down to produce two or more products. ...
Topic 1: Quantitative Chemistry
... Upon analysis, a sample of an acid with a molar mass of 194.13 g mol-1 was found to contain 0.25 g of hydrogen, 8.0 g of sulfur, and 16.0 g of oxygen. Determine the empirical and molecular formula. ...
... Upon analysis, a sample of an acid with a molar mass of 194.13 g mol-1 was found to contain 0.25 g of hydrogen, 8.0 g of sulfur, and 16.0 g of oxygen. Determine the empirical and molecular formula. ...
Chapter 6 ENZYME SUBSTRATE REACTANTS PRODUCTS
... 4. This term includes all the chemical reactions that allow cells to build and break down substances. Metabolism 5. This is the pocket in the enzyme into which the substrate bind. Active Site 6. Conditions such as extreme pH, temperature of salt cause enzymes to do this. Denature 7. This term descri ...
... 4. This term includes all the chemical reactions that allow cells to build and break down substances. Metabolism 5. This is the pocket in the enzyme into which the substrate bind. Active Site 6. Conditions such as extreme pH, temperature of salt cause enzymes to do this. Denature 7. This term descri ...
Unit 1 Ch. 2,3,4 notes NEW
... In a chemical reaction, the total mass of reactants always equals the total mass of products. eg. 2 Na3N → 6 Na + N2 When 500.00 g of Na3N decomposes 323.20 g of N2 is produced. How much Na is produced in this decomposition? 2:29 AM ...
... In a chemical reaction, the total mass of reactants always equals the total mass of products. eg. 2 Na3N → 6 Na + N2 When 500.00 g of Na3N decomposes 323.20 g of N2 is produced. How much Na is produced in this decomposition? 2:29 AM ...
c - SchoolRack
... About 25 of the 92 elements are essential to life Carbon, hydrogen, oxygen, and nitrogen make up 96% of living matter Most of the remaining 4% consists of calcium, phosphorus, potassium, and sulfur Trace elements are those required by an organism in minute quantities ...
... About 25 of the 92 elements are essential to life Carbon, hydrogen, oxygen, and nitrogen make up 96% of living matter Most of the remaining 4% consists of calcium, phosphorus, potassium, and sulfur Trace elements are those required by an organism in minute quantities ...
Stoichometry Notes (Unit 2)
... represents a chemical reaction in which two diatomic hydrogen (gas) molecules react with one diatomic oxygen (gas) molecule react to yield two water (liquid) molecules. Hydrogen and oxygen are the reactants (a.k.a. reagents) and water is the product. The “à” symbol separates the reactant(s) from the ...
... represents a chemical reaction in which two diatomic hydrogen (gas) molecules react with one diatomic oxygen (gas) molecule react to yield two water (liquid) molecules. Hydrogen and oxygen are the reactants (a.k.a. reagents) and water is the product. The “à” symbol separates the reactant(s) from the ...
Lecture 3: Reaction Tables and Limiting Reactants start with PRS
... H2(g) left over, there will be 50 O2(g) left over and 100 H2O(g) will be formed. This is an easy problem and this detailed treatment is not necessary in this case, but the general method we just used to solve the limiting reactant problem using a reaction table is a very powerful method that will h ...
... H2(g) left over, there will be 50 O2(g) left over and 100 H2O(g) will be formed. This is an easy problem and this detailed treatment is not necessary in this case, but the general method we just used to solve the limiting reactant problem using a reaction table is a very powerful method that will h ...
Atomic Structure Practice Test
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
EXAM 3
... A 5.000 g sample of a compound known to contain only the elements phosphorous and oxygen was analyzed and found to contain 2.182 g of phosphorous. Additional experiments indicate that this compound has a molecular weight of 283.9 g/mol. How many phosphorous atoms are present in each molecule of this ...
... A 5.000 g sample of a compound known to contain only the elements phosphorous and oxygen was analyzed and found to contain 2.182 g of phosphorous. Additional experiments indicate that this compound has a molecular weight of 283.9 g/mol. How many phosphorous atoms are present in each molecule of this ...
Science 10 (4.2) Names and formulas of
... of each element in the molecule is shown by the chemical formula. ...
... of each element in the molecule is shown by the chemical formula. ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.