Unit 13: Electrochemistry (Link to Prentice Hall Text: Chapters 22
... A car battery powers the car through a spontaneous reaction, but what can you do if the battery dies? (c) To coat one metal on top of another one, as with jewelry, or exhaust pipes. a. To make something look more expensive or shinier b. To improve corrosion resistance ...
... A car battery powers the car through a spontaneous reaction, but what can you do if the battery dies? (c) To coat one metal on top of another one, as with jewelry, or exhaust pipes. a. To make something look more expensive or shinier b. To improve corrosion resistance ...
Chapter 19 Chemical Thermodynamics
... Analyze: We are asked to judge whether each process will proceed spontaneously in the direction indicated, in the reverse direction, or in neither direction. Plan: We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect ...
... Analyze: We are asked to judge whether each process will proceed spontaneously in the direction indicated, in the reverse direction, or in neither direction. Plan: We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect ...
Chemical Equilibrium - Request a Spot account
... point or freezing point (constant temperatures) can experience a change in heat without experiencing a change in temperature. Most equilibrium systems are not studied at their boiling point or freezing point, though. Consequently, a change in heat generally corresponds to a change in temperature and ...
... point or freezing point (constant temperatures) can experience a change in heat without experiencing a change in temperature. Most equilibrium systems are not studied at their boiling point or freezing point, though. Consequently, a change in heat generally corresponds to a change in temperature and ...
PHYSICAL SETTING CHEMISTRY
... 77 What is the total number of moles of CO2(g) in cylinder A? [1] 78 Explain why the number of molecules of N2(g) in cylinder B is the same as the number of molecules of CO2(g) in cylinder A. [1] 79 The temperature of the CO2(g) is increased to 450. K and the volume of cylinder A remains constant. I ...
... 77 What is the total number of moles of CO2(g) in cylinder A? [1] 78 Explain why the number of molecules of N2(g) in cylinder B is the same as the number of molecules of CO2(g) in cylinder A. [1] 79 The temperature of the CO2(g) is increased to 450. K and the volume of cylinder A remains constant. I ...
MC94 - Southchemistry.com
... (B) Ca (C) Zn (D) As (E) Na 11. Utilized as a coating to protect Fe from corrosion 12. Is added to silicon to enhance its properties as a semiconductor 13. Utilized as a shield from sources of radiation Directions: Each of the questions or incomplete statements below is followed by five suggested an ...
... (B) Ca (C) Zn (D) As (E) Na 11. Utilized as a coating to protect Fe from corrosion 12. Is added to silicon to enhance its properties as a semiconductor 13. Utilized as a shield from sources of radiation Directions: Each of the questions or incomplete statements below is followed by five suggested an ...
Document
... The limiting reagent is the reactant you run out of first. The excess reagent is the one you have left over. The limiting reagent determines how much product you can make ...
... The limiting reagent is the reactant you run out of first. The excess reagent is the one you have left over. The limiting reagent determines how much product you can make ...
29th INTERNATIONAL CHEMISTRY OLYMPIAD PREPARATORY
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
IChO_Comp_Prob_Answ 1997
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
Net ionic equation
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
... The forces holding an ionic compound together are the strong electrical attraction that exists between cations and anions. It is therefore somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a par ...
Advanced Placement Chemistry
... 74. A solution of calcium hypochlorite, a common additive to swimming-pool water, is (A) basic because of the hydrolysis of the OCl¯ ion (B) basic because Ca(OH)2 is a weak and insoluble base (C) neutral if the concentration is kept below 0.1 molar (D) acidic because of the hydrolysis of the Ca2+ i ...
... 74. A solution of calcium hypochlorite, a common additive to swimming-pool water, is (A) basic because of the hydrolysis of the OCl¯ ion (B) basic because Ca(OH)2 is a weak and insoluble base (C) neutral if the concentration is kept below 0.1 molar (D) acidic because of the hydrolysis of the Ca2+ i ...
Reaction Kinetics - National Open University of Nigeria
... chemical reactions. The study of reaction rates allows for the prediction of how fast it will take a reaction mixture to reach equilibrium. It also account for how the reaction rate would be optimised by controlling certain factors such as temperature, pressure and the presence of a catalyst. The st ...
... chemical reactions. The study of reaction rates allows for the prediction of how fast it will take a reaction mixture to reach equilibrium. It also account for how the reaction rate would be optimised by controlling certain factors such as temperature, pressure and the presence of a catalyst. The st ...
1 - KFUPM Faculty List
... Al is a representative metallic element and its monoatomic ion is Al3+ (main group IIIa, 3 steps to the next smaller noble gas). Sulfite is SO32- (lower oxygen content), while SO42- is sulfate (higher oxygen content). Charge neutrality requires thus (Al3+)2(SO32-)3 = Al2(SO3)3 for aluminum sulfite g ...
... Al is a representative metallic element and its monoatomic ion is Al3+ (main group IIIa, 3 steps to the next smaller noble gas). Sulfite is SO32- (lower oxygen content), while SO42- is sulfate (higher oxygen content). Charge neutrality requires thus (Al3+)2(SO32-)3 = Al2(SO3)3 for aluminum sulfite g ...
Review: SS Nonisothermal Reactors - University of Illinois Urbana
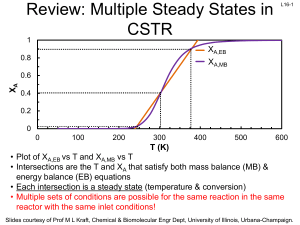
... Reaches steady state at ~12 minutes Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. ...
... Reaches steady state at ~12 minutes Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. ...
Analytical Chemistry
... M.wt. Na2CO3 = 23*2 +12*1 + 16*3 =106 g/mol. Wt. of Na2CO3 = 0.2 mol. * 106 g/mol = 21.2g Therefor, dissolve 21.2 g of the Na2CO3 in water and dilute to exactly 2.00 L. ...
... M.wt. Na2CO3 = 23*2 +12*1 + 16*3 =106 g/mol. Wt. of Na2CO3 = 0.2 mol. * 106 g/mol = 21.2g Therefor, dissolve 21.2 g of the Na2CO3 in water and dilute to exactly 2.00 L. ...
Redox Balancing Worksheet
... Oxidation-reduction (redox) reactions are reactions in which oxidation numbers change. Oxidation numbers are either real charges or formal charges which help chemists keep track of electron transfer. In practice, oxidation numbers are best viewed as a bookkeeping device. Oxidation cannot occur witho ...
... Oxidation-reduction (redox) reactions are reactions in which oxidation numbers change. Oxidation numbers are either real charges or formal charges which help chemists keep track of electron transfer. In practice, oxidation numbers are best viewed as a bookkeeping device. Oxidation cannot occur witho ...
Chemistry
... the atoms of metallic elements - lithium, sodium, magnesium, aluminum, potassium, calcium, iron; reactions equations which characterize chemical properties of the metals (interaction with oxygen, halogens, sulfur, water, solutions of acids, alkalis and salts) and processes of their preparation (oxid ...
... the atoms of metallic elements - lithium, sodium, magnesium, aluminum, potassium, calcium, iron; reactions equations which characterize chemical properties of the metals (interaction with oxygen, halogens, sulfur, water, solutions of acids, alkalis and salts) and processes of their preparation (oxid ...
111 Exam III OUTLINE TRO 1-3-11
... When equilibrium between substances involve two or more phases it is called Heterogeneous Equilibria. The concentration of a pure solid or a pure liquid in their standard states is constant (at constant T° and P). Therefore, the concentrations of solids or liquids involved in a heterogeneous equilib ...
... When equilibrium between substances involve two or more phases it is called Heterogeneous Equilibria. The concentration of a pure solid or a pure liquid in their standard states is constant (at constant T° and P). Therefore, the concentrations of solids or liquids involved in a heterogeneous equilib ...
Equilibrium and Pressure
... C. How does this value of Kp compare to the value you found before? ______________ ___________________________________________________________________ D. As the experiment reached equilibrium again, did the reactants or products increase? _____________________________________________________________ ...
... C. How does this value of Kp compare to the value you found before? ______________ ___________________________________________________________________ D. As the experiment reached equilibrium again, did the reactants or products increase? _____________________________________________________________ ...
Chemistry - Ysgol Bro Pedr
... You need to be able to give an example and a brief outline of a beneficial use of radioactivity in each of the following contexts: Health and Medicine We have just stated that radiation is harmful to health. However, it can be used successfully in a controlled way in order to treat and trace disease ...
... You need to be able to give an example and a brief outline of a beneficial use of radioactivity in each of the following contexts: Health and Medicine We have just stated that radiation is harmful to health. However, it can be used successfully in a controlled way in order to treat and trace disease ...
chem A exercise package C
... Many formulas for substances cannot be explained in terms of ionic bonding. Consider the substance Cl2O. Both the chlorine and the oxygen atom need more electrons for a stable electron population. A model proposed that would allow both atoms to gain electrons is shown in the diagram on this page. Th ...
... Many formulas for substances cannot be explained in terms of ionic bonding. Consider the substance Cl2O. Both the chlorine and the oxygen atom need more electrons for a stable electron population. A model proposed that would allow both atoms to gain electrons is shown in the diagram on this page. Th ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.