* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Review: SS Nonisothermal Reactors - University of Illinois Urbana
Survey
Document related concepts
Transcript
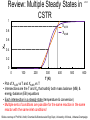
Review: Multiple Steady States in CSTR 1 L16-1 XA,EB XA,MB 0.8 XA 0.6 0.4 0.2 0 0 100 200 300 400 500 600 T (K) • Plot of XA,EB vs T and XA,MB vs T • Intersections are the T and XA that satisfy both mass balance (MB) & energy balance (EB) equations • Each intersection is a steady state (temperature & conversion) • Multiple sets of conditions are possible for the same reaction in the same reactor with the same inlet conditions! Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-2 Review: Heat Removal Term R(T) & T0 Heat removed: R(T) Heat generated G(T) r V Cp0 1 T TC HRX A FA0 R(T) line has slope of CP0(1+) UA Cp0FA0 Tc Ta T0 1 R(T) =∞ =0 R(T) Increase Increase T0 T When T0 increases, slope stays same & line shifts to right For Ta < T0 Ta T0 T When increases from lowering FA0 or increasing heat exchange, slope and x-intercept moves Ta<T0: x-intercept shifts left as ↑ Ta>T0: x-intercept shifts right as ↑ =0, then TC=T0 =∞, then TC=Ta Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-3 Review: CSTR Stability G(T) Tc R(T) Ta T0 UA Cp0FA0 1 R(T) > G(T) → T falls to T=SS1 G(T) > R(T) → T rises to T=SS1 G(T) > R(T) → T rises to T=SS3 2 Heat removed: R(T) 1 3 R(T) > G(T) →T falls to T=SS3 Heat generated G(T) rA V FA0 Cp0 1 T TC HRX T • Magnitude of G(T) to R(T) curve determines if reactor T will rise or fall • G(T) = R(T) intersection, equal rate of heat generation & removal, no change in T • G(T) > R(T) (G(T) line above R(T) on graph): rate of heat generation > heat removal, so reactor heats up until a steady state is reached • R(T) > G(T) (R(T) line above G(T) on graph): rate of heat generation < heat removal, so reactor cools off until a steady state is reached Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-4 L16: Unsteady State Nonisothermal Reactor Design Q Goal: develop EB for unsteady state reactor An open system (for example, CSTR) Fin Fout Hin Hout W dEsys dt Q Rate of rate of heat accumulation flow from = of energy in surroundings system to system dEsys dt W n FE i i i1 in Rate of work Rate of energy done by added to + system on system by surroundings mass flow in 0 steady state dEsys dt n FE i i i1 out Rate of energy leaving system by mass flow out 0 unsteady state Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-5 Change in System Energy with Time dEsys dt n Q W FE i i i1 n in FE i i i1 out n Energy of system is the sum of products of each species E sys NiEi specific energy Ei & the moles of each species: i1 n Ei Ui & Ui Hi PVi so: Esys Ni Hi PVi Differentiate wrt time i1 dEsys dt dEsys n dHi n dNi d n d n N H P N V i i i i Ni Hi PVi dt dt dt dt dt i1 i1 i1 i1 Total V dEsys dt n dHi n dNi d Ni Hi PV dt i1 dt dt i1 n n Q WS FH i i i1 n FH i i in i1 0 For well-mixed reactor with constant PV- variation out dHi n dNi Ni Hi dt i1 dt i1 Total Energy Balance for unsteady state, constant PV Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-6 Well-Mixed Reactors, Constant PV Total Energy Balance for unsteady state n n n dHi n dNi d Q WS FH Ni Hi PV = 0 i i FH i i dt i1 dt dt i1 in i1 out i1 Special case: well-mixed reactors (e.g., batch, CSTR or semibatch) with constant PV- variation in total P or V can be neglected n n Q WS FH i i i1 n FH i i in i1 out dHi n dNi Ni Hi dt i1 dt i1 Total Energy Balance for unsteady state, constant PV T dHi dHi dT Evaluate recalling that Hi H RX TR Cpi dT so Cpi dt dt dt T R n n Q WS FH i i i1 n FH i i in i1 out dT n dNi NiCpi Hi dt i1 dt i1 Need to put dNi/dt into terms that can be measured Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-7 EB for Well-Mixed Reactors, PV=0 n n Q WS FH i i i1 n FH i i in i1 out dT n dNi NiCpi Hi dt i1 dt i1 From the mass balance: Accumulation = In - Out + Gen dNi dNi Fi0 F i irA V Substitute Fi0 F i i rA V dt dt n n n dT n Q WS FH NiCpi Hi Fi0 F i irA V i i FH i i dt i1 i1 i1 in i1 out n n n n n dT n Q WS Fi0Hi0 FH HiFi0 HiFi iHi rA V i i NiCpi dt i1 i1 i1 i1 i1 i1 Add SFiHi to both sides of equation: n n n dT n Q WS Fi0Hi0 NiCpi HiFi0 iHi rA V dt i1 i1 i1 i1 Substitute ΣiHi =H°RX(T): n n H°RX(T) dT n Q WS Fi0Hi0 NiCpi HiFi0 H RX T rA V dt i1 i1 i1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-8 Simplified EB for Well-Mixed Reactors n n dT n HF Solve for dT/dt: Q WS Fi0Hi0 NiCpi i i0 H RX T rA V dt i1 i1 i1 Bring SFi0Hi and H°RX(T) terms to other side of equation: n n n dT Q WS Fi0Hi0 HF i i0 H RX T rA V NiCpi dt i1 i1 i1 Factor SFi0Hi0 and SFi0Hi terms and divide by SNiCpi : n Energy balance for unsteady state reactor with phase change: Q WS Fi0 Hi Hi0 H RX T rA V i1 n NiCpi dT dt i1 n Energy balance for Q WS Fi0Cpi T Ti0 H RX T rA V dT i1 unsteady state reactor n dt NiCpi without phase change: i1 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-9 Unsteady State EB, Liquid-Phase Rxns n Q WS Fi0 Cpi T Ti0 H RX T rA V i1 n NiCpi dT dt i1 For liquid-phase reactions, often Cp = SiCpi is so small it can be neglected When Cp can be neglected, then: n NiCpi NA0 Cps where Cps SiCpi is the heat capacity of the solution i1 If the feed is well-mixed, it is convenient to use: SFi0 Cpi FA0 Cps Plug these equations and Ti0 = T0 into the EB gives: Q WS FA 0 Cps T Ti0 H RX T rA V dT NA0 Cps dt This equation for the EB is simultaneously solved with the mass balance (design eq) for unsteady state, nonisothermal reactor design Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-10 Nonisothermal Batch Reactor Design n Q WS Fi0Cpi T Ti0 H RX T rA V i1 0 n NiCpi dT dt i1 No flow, so: Q WS H RX T rA V n NiCpi dT dt i1 Put the energy balance in terms of XA: Ni NA0 i i X A Ni0 where i & iCpi CP NA 0 Q WS H RX T rA V dT n dt NA0 iCpi Cp X A i1 Solve with the batch reactor design equation using an ODE solver (Polymath) dX A NA0 rA V dt Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Adiabatic Nonisothermal Batch Reactor Design 0 0 L16-11 Q WS H RX T rA V dT n dt NA0 iCpi Cp X A i1 In the case of no stirring work and adiabatic operation, WS =0 & Q 0 H RX T rA V n NA0 iCpi Cp X A i1 dT Substitute: H RX T rA V dT dt iCpi Cps NA0 Cps Cp X A dt dT dt dX A H T H T C T T an d -N rA V Substitute: RX RX R p R A0 dt dX A dT H RX TR Cp T TR NA 0 NA0 CpS Cp X A dt dt dX dT H RX TR Cp T TR A CpS Cp X A dt dt Rearrange: H RX T rA V NA0 CpS Cp X A Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. dX A dT Solve for how XA H T C T T C C X RX R p R p A pS dt dt changes with T L16-12 H RX TR Cp T TR dX A CpS Cp X A dT Get like terms together: XA X A0 0 CpS T dX A dT Cp X A T H RX TR Cp T TR 0 Integrate & solve for XA: XA T T0 XA H RX TR Cp T TR Cp S T T0 HRX T Cp S Solving for T: H RX T0 X A H RX T0 X A T T0 T T0 n Cp X A Cp s iCp X A Cp i1 Heat capacity of soln (calculate Cps if not given) i Solve with the batch reactor design equation using an ODE solver (Polymath) XA t NA0 0 dX A rA V Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-13 1st A order, liquid-phase, exothermic reaction A→B is run in a batch reactor. The reactor is well-insulated, so no heat is lost to the surroundings. To control the temperature, an inert liquid C is added to the reaction. The flow rate of C is adjusted to keep T constant at 100 °F. What is the flow rate of C after 2h? TC0 = 80 °F V0= 50 ft3 H°RX=-25000 Btu/lb mol k(100 °F)= 1.2 x 10-4 s-1 Cp, (all components)= 0.5 Btu/lb mol °F CA0= 0.5 lb mol/ft3 1. Solve design eq for comp as function of t 2. Solve EB for FC0 using that info & T=100 °F This is essentially a semi-batch reactor since only C is fed into the reactor dX A dNA rA V would complicate Design eq: rA V Note, using NA0 d t dt the calculation because V depends on t Rate eq: -rA = kCA dNA dNA kC V kNA Rearrange and integrate for NA Combine: A dt dt NA dNA t ln kdt kt NA NA0 exp kt NA0 NA0 NA 0 NA Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-14 1st A order, liquid-phase, exothermic reaction A→B is run in a batch reactor. The reactor is well-insulated, so no heat is lost to the surroundings. To control the temperature, an inert liquid C is added to the reaction. The flow rate of C is adjusted to keep T constant at 100 °F. What is the flow rate of C after 2h? Use EB to find how the flow rate of C depends on the rxn (solve EB for FC0) 0 0 n Q WS Fi0 Cpi T Ti0 H RX T rA V dT i1 n dt NiCpi 0 i1 n n i1 i1 0 Fi0Cpi T Ti0 H RX T rA V Fi0 Cpi T Ti0 H RX T rA V C is the only species that flows, so: FC0 CpC T Ti0 H RX T rA V rAV = -kCAV = -kNA where NA NA0 exp kt FC0 CpC T Ti0 H RX T kNA0 ekt Isolate FC0: FC0 H RX T kNA0 ekt CpC T Ti0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-15 1st A order, liquid-phase, exothermic reaction A→B is run in a batch reactor. The reactor is well-insulated, so no heat is lost to the surroundings. To control the temperature, an inert liquid C is added to the reaction. The flow rate of C is adjusted to keep T constant at 100 °F. What is the flow rate of C after 2h? H°RX=-25000 Btu/lb mol Cp, (all components)= 0.5 Btu/lb mol °F TC0 = 80 °F V0= 50 ft3 k(100 °F)= 1.2 x 10-4 s-1 CA0= 0.5 lb mol/ft3 FC0 H RX T kNA 0 ekt CpC T Ti0 NA0 CA0 V0 0.5 At 2h (7200s): FC0 lb mol ft 3 1.210 Btu 4 1 1.2 10 s 25 lb mol e 25,000 lb mol Btu 0.5 100 F 80 F lb mol F FC0 3.16 50ft 3 25 lb mol 4 s1 7200s lb mol s Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-16 Instead of feeding coolant to the reactor, a solvent with a low boiling point is added (component D). The solvent has a heat of vaporization of 1000 Btu/lb mol, and initially 25 lb mol of A are placed in the tank. The reactor is wellinsulated. What is the rate of solvent evaporation after 2 h if T is constant at 100 °F? Additional info: k(100 °F)= 1.2 x 10-4 s-1 H°RX=-25000 Btu/lb mol Still a semibatch reactor, where D is removed from the reactor Use EB that accounts for a phase change: 0 0 n Q WS Fi0 Hi0 Hi H RX T rA V Q̇ =0 dT i1 ẆS=0 n dt NiCpi 0 i1 Clicker Question: Does dT/dt = 0? a) Yes b) No Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-17 Instead of feeding coolant to the reactor, a solvent with a low boiling point is added (component D). The solvent has a heat of vaporization of 1000 Btu/lb mol, and initially 25 lb mol of A are placed in the tank. What is the rate of solvent evaporation after 2 h? Additional info: k(100 °F)= 1.2 x 10-4 s-1 H°RX=-25000 Btu/lb mol Still a semibatch reactor, where D is removed from the reactor Use EB that accounts for a phase change: 0 0 n Q WS Fi0 Hi0 Hi H RX T rA V Q̇ =0 dT i1 ẆS=0 n dt NiCpi dT/dt = 0 0 i1 n Fi0 Hi0 Hi H RX T rA V i1 D is the only species that ‘flows’, and rAV = -kNA0(exp[-kt]), so: FD0 Hi0 Hi H RX T kNA 0 ekt F D0 Hi0-Hi = heat of vap 25,000 FD0 H RX T kNA0 ekt Hi0 Hi Btu 0.00012 25 lb mol e 0.00012 s 7200s F 0.0316 lb mol lbmol s D0 s 1000Btu lb mol Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. A liquid phase exothermic reaction A →B is carried out at 358K in a 0.2 m3 CSTR. L16-18 The coolant temperature is 273K and the heat transfer coefficient (U) is 7200 J/min·m2·K. What is the heat exchange area required for steady state operation? Using this heat exchange area, plot T vs t for reactor start-up. CPA =CPS=20 J/g•K ẆS=0 CA0= 180 g/dm3 u0= 500 dm3/min T0= 313 K r= 900 g/dm3 H°RX(T) = -2500 J/g E=94852 J/mol·K k(313K)= 1.1 min-1 a) Heat exchange area for steady state operation: SS operation means that T is constant, so: n dT dt Q WS Fi0Cpi T Ti0 H RX T rA V i1 n 0 NiCpi n i1 Q WS Fi0 Cpi T Ti0 H RX T rA V i1 Q̇ =UA(Ta-T), ẆS=0, and A is only species that flows UA Ta T FA0 CpA T TA0 H RX T rA V Plug in rA = -kCA and solve for A UA Ta T FA0 CpA T TA0 H RX T kC A V A H RX T kC A V FA0 CpA T TA0 U Ta T Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. A liquid phase exothermic reaction A →B is carried out at 358K in a 0.2 m3 CSTR. L16-19 The coolant temperature is 273K and the heat transfer coefficient (U) is 7200 J/min·m2·K. What is the heat exchange area required for steady state operation? Using this heat exchange area, plot T vs t for reactor start-up. CPA =CPS=20 J/g•K ẆS=0 CA0= 180 g/dm3 u0= 500 dm3/min T0= 313 K r= 900 g/dm3 H°RX(T) = -2500 J/g E=94852 J/mol·K k(313K)= 1.1 min-1 Use material balance to determine steady state value of CA FA0 FA rA V 0 C A0u0 CAu0 kC A V 0 CA0u0 CAu0 kCA V 1.1 1 94852 J mol 1 C A0u0 107.4 exp C A k 358K = k 358K min 8.314 J mol K 313 358 u0 kV min CA 180 g dm3 500 dm3 min 500 dm3 min 107.4min1 200dm3 Solve EB for A: A C A 4.1g dm3 H RX T kC A V FA0 CpA T TA0 g dm3 FA0 180 500 90000 g min 3 min dm U Ta T 3 3 1000dm V=0.2m 3 m 3 200dm Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. A liquid phase exothermic reaction A →B is carried out at 358K in a 0.2 m3 CSTR. L16-20 The coolant temperature is 273K and the heat transfer coefficient (U) is 7200 J/min·m2·K. What is the heat exchange area required for steady state operation? Using this heat exchange area, plot T vs t for reactor start-up. CPA =CPS=20 J/g•K ẆS=0 CA0= 180 g/dm3 u0= 500 dm3/min T0= 313 K r= 900 g/dm3 H°RX(T) = -2500 J/g E=94852 J/mol·K k(313K)= 1.1 min-1 Solve for heat exchange area at SS: A H RX T kC A V FA 0 CpA T TA 0 U Ta T FA0=90,000 g/min H°RX(T) = -2500 J/g U= 7200 J/min·m2·K V=200 dm3 k= 107.4 min-1 CA= 4.1 g/dm3 CPA =20 J/g•K TA0= 313 K T= 358K Ta=273K J 107.4 g g J 3 2500 4.1 200dm 90000 20 358K 313K 3 g m in m i n g K dm A J 7 200 273K 358K 2 min m K A=227.4 m2 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. Will use Polymath to plot T vs t for CSTR start-up (unsteady-state). Need equations for dCA/dt, dT/dt, and k. dNA dC A C A0 C A rA FA0 FA rA V Mass balance: dt t t dt L16-21 dT UA Ta T FA0CpA T Ti0 H RX rA V n dt Ni0Cps i1 CPs is in terms of mass (J/g·K), so FA0 & Ni0 must also be in terms of mass FA0=90,000 g/min Substitute ṁi0 for Ni0, & use r for the solution to calculate: g 3 m 200d m 9 0 0 mi0 180,000g mi0 Vr i0 3 dm rA=-kCA Amount of gas leaving reactor (L7) 1.1 1 94852J mol 1 1.1 1 11408.7 1 k= exp k= exp K min min 313 T 8.314 J mol K 313 T U= 7200 J/min·m2·K A=227.4m2 CA0=180g/dm3 HRX=-2500 J/g Ni0=mi0 V=200 dm3 CPs=20 J/g˙K t=V/u0 Ta=273K u0=500 dm3 T0=313 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-22 Will use Polymath to plot T vs t for CSTR start-up (unsteady-state). Need equations for dCA/dt, dT/dt, and k. 1.1 1 11408.7 1 dC A C A0 C A k= exp rA=-kCA rA min dt t t 313 T K dT UA Ta T FA0CpA T Ti0 H RX rA V mi0 180,000g n dt Ni0Cps F =90,000 g/min i1 U= 7200 J/min·m2·K A=227.4m2 CA0=180g/dm3 HRX=-2500 J/g Ni0=mi0 V=200 dm3 CPs=20 J/g˙K A0 t=V/u0 Ta=273K u0=500 dm3 T0=313 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. T (K) L16-23 t (min) Reaches steady state at ~12 minutes Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-24 The elementary, liquid phase, exothermic reaction A →B is carried out in a 2 m3 CSTR that is equipped with a heat jacket. Pure A enters the reactor at 60 mol/min and a temperature of 310K. The coolant in the heat jacket is kept at 280 K. Provide all equations, all constants, the initial time, and the final time that must be entered into Polymath in order to plot temperature vs time for the first 20 min of reactor start-up. ΔHRX(TR) = -10,000 cal/mol CPA = CPS =15 cal/mol·K CpB=15 cal/mol·K ẆS=0 E = 20,000 cal/mol k = 1 min-1 at 400 K UA= 3200 cal/min•K u0= 300 L/min Need equations for how T changes with time, CA changes with time, & k changes with T. n dT dt Q WS Fi0 Cpi T Ti0 H RX T rA V i1 n WS 0 Q UA T - T a NiCpi i1 HRX T H RX TR CP T TR CP CPB CPA Cp 15 15 b a cal 0 mol K cal H T 10,000 H RX T H RX TR 0 RX mol n F C i 1 i0 pi n FA 0 Cp,A FB0 Cp,B FB0 0 Fi0 Cpi FA0 Cp,A i 1 n NiCpi NA0 Cps i1 UA T - T FA 0 Cp,A T Ti0 H T r V a RX R A Combine with EB: dT dt NA0 Cps Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign. L16-25 The elementary, liquid phase, exothermic reaction A →B is carried out in a 2 m3 CSTR that is equipped with a heat jacket. Pure A enters the reactor at 60 mol/min and a temperature of 310K. The coolant in the heat jacket is kept at 280 K. Provide all equations, all constants, the initial time, and the final time that must be entered into Polymath in order to plot temperature vs time for the first 20 min of reactor start-up. ΔHRX(TR) = -10,000 cal/mol CPA = CPS =15 cal/mol·K CpB=15 cal/mol·K ẆS=0 E = 20,000 cal/mol k = 1 min-1 at 400 K UA= 3200 cal/min•K u0= 300 L/min Need equations for how T changes with time, CA changes with time, & k changes with T. cal UA T - T FA 0 Cp,A T Ti0 H T r V H RX T 10,000 dT a RX R A mol UA 3200 FA0 60 FA0 Ta 280 CPA 15 dt t f 20 NA0 Cps Ti0 310 FA 0 V NA 0 u0 NA0 u0 V t 0 0 rA kC A u0 300 CPS 15 20,000 1 1 k 1 exp 400 T 1.987 V 2m3 1000L 1m3 V 2000 Use the mass balance to get eq for CA(t) dC A C A0 C A dNA dNA kC A C u C u k C V FA0 FA rA V A0 0 A 0 A dt t t dt dt t V u0 C A0 FA 0 u0 Slides courtesy of Prof M L Kraft, Chemical & Biomolecular Engr Dept, University of Illinois, Urbana-Champaign.