Writing Equilibrium Cons... and Liquids - Chemwiki
... No state symbols have been given, but they will be all (g), or all (l), or all (aq) if the reaction was between substances in solution in water. If you allow this reaction to reach equilibrium and then measure the equilibrium concentrations of everything, you can combine these concentrations into an ...
... No state symbols have been given, but they will be all (g), or all (l), or all (aq) if the reaction was between substances in solution in water. If you allow this reaction to reach equilibrium and then measure the equilibrium concentrations of everything, you can combine these concentrations into an ...
chapter 16
... You must check validity by plugging "x" over original concentration. It must be less than 5% of the original concentration to be valid. 2. If "x" is necessary, then see if the problem may be a perfect square and thus, ease the steps of solving. (Sometimes you must use the quadratic formula!) 3. If n ...
... You must check validity by plugging "x" over original concentration. It must be less than 5% of the original concentration to be valid. 2. If "x" is necessary, then see if the problem may be a perfect square and thus, ease the steps of solving. (Sometimes you must use the quadratic formula!) 3. If n ...
KCl + O KClO 3 → However, this equation is not balanced, since
... chemical reaction as a reactant must appear in the products of the reaction. Atoms are neither created nor destroyed in ordinary chemical reactions, but the various atoms must be rearranged into new compounds in order to have any chemical reaction at all. The process of adjusting the numbers of each ...
... chemical reaction as a reactant must appear in the products of the reaction. Atoms are neither created nor destroyed in ordinary chemical reactions, but the various atoms must be rearranged into new compounds in order to have any chemical reaction at all. The process of adjusting the numbers of each ...
Chapter 4 Lecture Notes in PowerPoint
... • For reactions with multiple reactants, it is likely that one of the reactants will be completely used before the others. • When this reactant is used up, the reaction stops and no more product is made. • The reactant that limits the amount of product is called the limiting reactant. – It is someti ...
... • For reactions with multiple reactants, it is likely that one of the reactants will be completely used before the others. • When this reactant is used up, the reaction stops and no more product is made. • The reactant that limits the amount of product is called the limiting reactant. – It is someti ...
Mastering Medicinal Chemistry Brochure
... Stewart L. Fisher, Ph.D., Principal, SL Fisher Consulting, LLC Peter J. Tonge, Ph.D., Professor, Chemistry, Institute for Chemical Biology & Drug Discovery, Stony Brook University Participants will be introduced to the fundamentals of time-dependent inhibition and how these factors can impact drug d ...
... Stewart L. Fisher, Ph.D., Principal, SL Fisher Consulting, LLC Peter J. Tonge, Ph.D., Professor, Chemistry, Institute for Chemical Biology & Drug Discovery, Stony Brook University Participants will be introduced to the fundamentals of time-dependent inhibition and how these factors can impact drug d ...
12 - einstein classes
... The trihalides are predominantly covalent and, like NH3, have a tetrahedral structure with one position occupied by a lone pair. The exceptions are BiF3 which is ionic and the other halides of Bi and SbF3 which are intermediate in character. The trihalides typically hydrolyse readily with water, but ...
... The trihalides are predominantly covalent and, like NH3, have a tetrahedral structure with one position occupied by a lone pair. The exceptions are BiF3 which is ionic and the other halides of Bi and SbF3 which are intermediate in character. The trihalides typically hydrolyse readily with water, but ...
AP Chemistry - Notes
... b. conservation of atoms (mass) - atoms are neither created nor destroyed in chemical reactions, they are recombined to form different substances - mass is neither created nor destroyed chemical reactions (as opposed to nuclear reactions) - chemical reactions must therefore be balanced - have same k ...
... b. conservation of atoms (mass) - atoms are neither created nor destroyed in chemical reactions, they are recombined to form different substances - mass is neither created nor destroyed chemical reactions (as opposed to nuclear reactions) - chemical reactions must therefore be balanced - have same k ...
Q - PIMS
... The substance whose analysis is required for the separation of isotopes is converted into vapours. The pressure of vapours is reduced to 106—107 torr. These vapours at low pressure are allowed to enter the ionization chamber. (ii) Ionization chamber: In this chamber fast moving electrons are bomba ...
... The substance whose analysis is required for the separation of isotopes is converted into vapours. The pressure of vapours is reduced to 106—107 torr. These vapours at low pressure are allowed to enter the ionization chamber. (ii) Ionization chamber: In this chamber fast moving electrons are bomba ...
Higher Chemistry Resources Guide - Glow Blogs
... classic experiments such as the floating needle on the surface of a glass of water, or adding coins to a wine glass full of water to demonstrate the level rising above the rim of the glass. Hydrogen bonding is at the heart of 'hydrogel' materials. Examples of which are ...
... classic experiments such as the floating needle on the surface of a glass of water, or adding coins to a wine glass full of water to demonstrate the level rising above the rim of the glass. Hydrogen bonding is at the heart of 'hydrogel' materials. Examples of which are ...
å¾è湿çå¦
... 13 An isotope of a particular element has a mass number of 208 and it has 46 more neutrons than protons. What is the element? ...
... 13 An isotope of a particular element has a mass number of 208 and it has 46 more neutrons than protons. What is the element? ...
2 - cloudfront.net
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
Problem 1-2 - IPN-Kiel
... v) Does the formation of Fe3O4 lead to a higher or to a lower calculated content of iron? Account for your answer. vi) Calculate the mass of the iron(III) chloride sample which was given into the measuring flask. ...
... v) Does the formation of Fe3O4 lead to a higher or to a lower calculated content of iron? Account for your answer. vi) Calculate the mass of the iron(III) chloride sample which was given into the measuring flask. ...
An Analogy for an Equilibrium Reaction
... What is the value of Keq at 395°C for the reaction COCl2(g) ⇌ CO(g) + Cl2(g) (The reverse reaction)? ...
... What is the value of Keq at 395°C for the reaction COCl2(g) ⇌ CO(g) + Cl2(g) (The reverse reaction)? ...
CHAPTER 9 CHEMICAL BONDING I
... In the third period of the periodic table and beyond, atoms have d orbitals in addition to the s and p orbitals that can be used in bonding. These orbitals enable an atom to form an expanded octet. ...
... In the third period of the periodic table and beyond, atoms have d orbitals in addition to the s and p orbitals that can be used in bonding. These orbitals enable an atom to form an expanded octet. ...
Groups 2 and 7
... Electronegativity of the halogens decreases down the group due to an increase in atomic radius. Increased nuclear charge has no significant effect because there are more electron shells and more shielding. Iodine atoms therefore attract electron density in a covalent bond less strongly than fluorine ...
... Electronegativity of the halogens decreases down the group due to an increase in atomic radius. Increased nuclear charge has no significant effect because there are more electron shells and more shielding. Iodine atoms therefore attract electron density in a covalent bond less strongly than fluorine ...
1. Atomic Structure and Periodic Table THE MASS SPECTROMETER
... further from the nucleus and are more shielded so the attraction of the nucleus becomes smaller Q. Why is there a general increase in first ionisation energy across a period? A. As one goes across a period , the number of protons increases making the effective attraction of the nucleus greater. The ...
... further from the nucleus and are more shielded so the attraction of the nucleus becomes smaller Q. Why is there a general increase in first ionisation energy across a period? A. As one goes across a period , the number of protons increases making the effective attraction of the nucleus greater. The ...
Solution - HCC Learning Web
... Check Notice that the net charge in the diagram is zero, as it must be if it is to represent an ionic substance. ...
... Check Notice that the net charge in the diagram is zero, as it must be if it is to represent an ionic substance. ...
Chapter 10
... If you start with 3.00 mol of sulfur and 2.50 mol of sulfur reacts to produce FeS, you have 0.50 mol of excess sulfur (3.00 mol – 2.50 mol). The table below summarizes the amounts of each substance before and after the reaction. ...
... If you start with 3.00 mol of sulfur and 2.50 mol of sulfur reacts to produce FeS, you have 0.50 mol of excess sulfur (3.00 mol – 2.50 mol). The table below summarizes the amounts of each substance before and after the reaction. ...
Thermochemistry
... and Its Transformations The month may vary, depending on whether you live in Maine or Texas, but for most people, the annual fall ritual is pretty much the same. The days get shorter, the temperature drops, and soon it is time to light the household furnace. The furnace at my house, like many, is fu ...
... and Its Transformations The month may vary, depending on whether you live in Maine or Texas, but for most people, the annual fall ritual is pretty much the same. The days get shorter, the temperature drops, and soon it is time to light the household furnace. The furnace at my house, like many, is fu ...
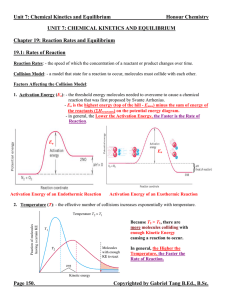
Unit 7 Reaction Rates and Equilibrium Notes
... Le Châtelier’s Principle: - a qualitative method to predict the shift on an equilibrium system if it is disturbed by means of changing concentration, pressure and temperature. - the equilibrium will shift in the direction that minimizes the change imposed on the system. 1. Effects of a Change in Con ...
... Le Châtelier’s Principle: - a qualitative method to predict the shift on an equilibrium system if it is disturbed by means of changing concentration, pressure and temperature. - the equilibrium will shift in the direction that minimizes the change imposed on the system. 1. Effects of a Change in Con ...
N5 Chemistry 2014
... 1. The answer to each question is either A, B, C or D. Decide what your answer is, then fill in the appropriate bubble (see sample question below). 2. There is only one correct answer to each question. 3. Any rough work must be written in the additional space for answers and rough work at t ...
... 1. The answer to each question is either A, B, C or D. Decide what your answer is, then fill in the appropriate bubble (see sample question below). 2. There is only one correct answer to each question. 3. Any rough work must be written in the additional space for answers and rough work at t ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.