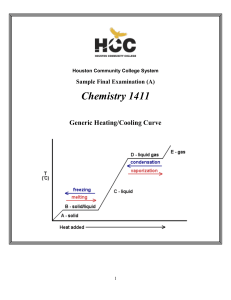
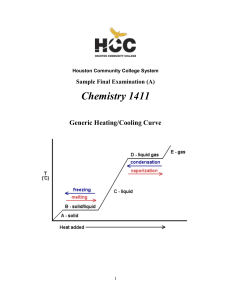
Oxidation and Reduction
... E. HCl(g) + H2O(l) → H3O+(aq) + Cl–(aq) F. 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l) ...
... E. HCl(g) + H2O(l) → H3O+(aq) + Cl–(aq) F. 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l) ...
Solution - Georgetown Independent School District
... Internal Energy, Heat, and Work A balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 4.00 × 106 L to 4.50 × 106 L by the addition of 1.3 × 108 J of energy as heat. ...
... Internal Energy, Heat, and Work A balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 4.00 × 106 L to 4.50 × 106 L by the addition of 1.3 × 108 J of energy as heat. ...
Wizard Test Maker
... conductor of electricity? A 3 A KCl B 7 B C6H12O6 C 8 C CO2 D 11 D CO 33. When ethylene glycol (an antifreeze) is added to water, what happens to the boiling point of the water? A It decreases, and the freezing point ...
... conductor of electricity? A 3 A KCl B 7 B C6H12O6 C 8 C CO2 D 11 D CO 33. When ethylene glycol (an antifreeze) is added to water, what happens to the boiling point of the water? A It decreases, and the freezing point ...
Slide 1
... which ions are leaving the surface is exactly equal to the rate at which they are joining it again. At that point there will be a constant negative charge on the magnesium, and a constant number of magnesium ions present in the solution around it. ...
... which ions are leaving the surface is exactly equal to the rate at which they are joining it again. At that point there will be a constant negative charge on the magnesium, and a constant number of magnesium ions present in the solution around it. ...
www.XtremePapers.com
... Molecules move without interacting with one another except for collisions. ...
... Molecules move without interacting with one another except for collisions. ...
PowerPoint - Science Geek
... Stoichiometry “In solving a problem of this sort, the grand thing is to be able to reason backward. This is a very useful accomplishment, and a very easy one, but people do not practice it much.” Sherlock Holmes, in Sir Arthur Conan Doyle’s A Study in Scarlet ...
... Stoichiometry “In solving a problem of this sort, the grand thing is to be able to reason backward. This is a very useful accomplishment, and a very easy one, but people do not practice it much.” Sherlock Holmes, in Sir Arthur Conan Doyle’s A Study in Scarlet ...
HighFour Chemistry Round 1 Category C: Grades 9 – 10 Thursday
... The use of calculator is required. Answer #11: Explanation: ...
... The use of calculator is required. Answer #11: Explanation: ...
CHEM-1411 Final Practice Exam
... second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electrons), but not sp, sp2, or sp3. Furthermore, to form a -bond, we must have unhybridized p-orbitals on the sulfu ...
... second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electrons), but not sp, sp2, or sp3. Furthermore, to form a -bond, we must have unhybridized p-orbitals on the sulfu ...
1411FINALSAMPLE+KEY - Houston Community College
... second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electrons), but not sp, sp2, or sp3. Furthermore, to form a -bond, we must have unhybridized p-orbitals on the sulfu ...
... second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electrons), but not sp, sp2, or sp3. Furthermore, to form a -bond, we must have unhybridized p-orbitals on the sulfu ...
Revised Higher 2014 Paper
... 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number) and Centre Name printed on it. Do not change any of these details. 4 If any of ...
... 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number) and Centre Name printed on it. Do not change any of these details. 4 If any of ...
Sample Exercise 19.1 Identifying Spontaneous Processes
... Analyze: We are asked to judge whether each process will proceed spontaneously in the direction indicated, in the reverse direction, or in neither direction. Plan: We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect ...
... Analyze: We are asked to judge whether each process will proceed spontaneously in the direction indicated, in the reverse direction, or in neither direction. Plan: We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect ...
Nikolai N. Semenov - Nobel Lecture
... theory of structure. Unfortunately, these rules were in most cases only qualitative; they had many exceptions and were often dependent upon the test conditions. Nowadays, roughly a hundred years since the advent of the theory of structure, questions of reaction velocity are dealt with from an electr ...
... theory of structure. Unfortunately, these rules were in most cases only qualitative; they had many exceptions and were often dependent upon the test conditions. Nowadays, roughly a hundred years since the advent of the theory of structure, questions of reaction velocity are dealt with from an electr ...
Thermodynamics - WordPress.com
... During phase changes the temp remains constant (even though heat is being added or removed from the system) so no change in the average kinetic energy (Ek) of the molecules occurs. According to the chemical bonding theory energy is required to overcome forces or bonds that hold particles together, s ...
... During phase changes the temp remains constant (even though heat is being added or removed from the system) so no change in the average kinetic energy (Ek) of the molecules occurs. According to the chemical bonding theory energy is required to overcome forces or bonds that hold particles together, s ...
Chemistry1100 Practice Exam 4 Choose the best answer for
... are allowed to react with 1.818 moles of O2, and this is the only reaction which occurs, what is the maximum quantity of carbon dioxide that could be produced? a. 1.447 moles b. 1.454 moles c. 1.456 moles d. 2.180 moles e. 0.3978 moles 13. Given the balanced chemical equation, C4H4 + 5 O2 → 4 CO2 + ...
... are allowed to react with 1.818 moles of O2, and this is the only reaction which occurs, what is the maximum quantity of carbon dioxide that could be produced? a. 1.447 moles b. 1.454 moles c. 1.456 moles d. 2.180 moles e. 0.3978 moles 13. Given the balanced chemical equation, C4H4 + 5 O2 → 4 CO2 + ...
Document
... Summarizing Limiting Reactant and Yield • The limiting reactant (or limiting reagent) is the reactant that is completely consumed in a chemical reaction and limits the amount of product. • The reactant in excess is any reactant that occurs in a quantity greater than is required to completely react ...
... Summarizing Limiting Reactant and Yield • The limiting reactant (or limiting reagent) is the reactant that is completely consumed in a chemical reaction and limits the amount of product. • The reactant in excess is any reactant that occurs in a quantity greater than is required to completely react ...
AP Chemistry
... C) Single Displacement (Redox) An element reacts with a compound totake the place of one of the elements of that compound. A new element is formed along with a new compound. a) Metal and Acid hydrogen + salt H2SO4(aq) + Fe(s) → FeSO4(aq) + H2(g) b) Metal and Water hydrogen + metal hydroxide OR me ...
... C) Single Displacement (Redox) An element reacts with a compound totake the place of one of the elements of that compound. A new element is formed along with a new compound. a) Metal and Acid hydrogen + salt H2SO4(aq) + Fe(s) → FeSO4(aq) + H2(g) b) Metal and Water hydrogen + metal hydroxide OR me ...
lec09 - McMaster Chemistry
... quickly (or slowly) a reaction will occur. • To predict if a reaction can occur at a reasonable rate, one needs to consider: KINETICS 3 Nov 97 ...
... quickly (or slowly) a reaction will occur. • To predict if a reaction can occur at a reasonable rate, one needs to consider: KINETICS 3 Nov 97 ...
Mathematical Operations
... On some calculators the display will show 5.8, then a space, followed by 03, the exponent. On other calculators, a small10 is shown with an exponent 3. ...
... On some calculators the display will show 5.8, then a space, followed by 03, the exponent. On other calculators, a small10 is shown with an exponent 3. ...
mass-mass problems.
... How to solve limiting reactant problems: In this type of problem, you will be given masses of two different reactants and asked to determine which is the limiting reagent and how much of a given product is produced. Do a mass-mass problems starting with reactant 1 and looking for the mass of the as ...
... How to solve limiting reactant problems: In this type of problem, you will be given masses of two different reactants and asked to determine which is the limiting reagent and how much of a given product is produced. Do a mass-mass problems starting with reactant 1 and looking for the mass of the as ...
Slide 1
... Analyze: We are asked to judge whether each process will proceed spontaneously in the direction indicated, in the reverse direction, or in neither direction. Plan: We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect ...
... Analyze: We are asked to judge whether each process will proceed spontaneously in the direction indicated, in the reverse direction, or in neither direction. Plan: We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect ...
19 BROWN Chemical Thermodynamics PPTSExercise
... Analyze: We are asked to judge whether each process will proceed spontaneously in the direction indicated, in the reverse direction, or in neither direction. Plan: We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect ...
... Analyze: We are asked to judge whether each process will proceed spontaneously in the direction indicated, in the reverse direction, or in neither direction. Plan: We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.