Review for Physical Science Test #2
... 1. Compounds are made of ______________________ of elements that are _______________________________ together. 2. What are two ways that atoms can be bonded together? (Hint: both have to do with electrons.) ...
... 1. Compounds are made of ______________________ of elements that are _______________________________ together. 2. What are two ways that atoms can be bonded together? (Hint: both have to do with electrons.) ...
Thermodynamics - Shailendra Kumar Chemistry
... reaction is most correct? a. ∆G° remains constant while ∆G changes and becomes equal to ∆G° at equilibrium. b. Both ∆G° and ∆G remain constant during a chemical reaction. c. Initially both ∆G and ∆G° are equal to zero. The value of ∆G° changes to a value determined by the equilibrium constant; the v ...
... reaction is most correct? a. ∆G° remains constant while ∆G changes and becomes equal to ∆G° at equilibrium. b. Both ∆G° and ∆G remain constant during a chemical reaction. c. Initially both ∆G and ∆G° are equal to zero. The value of ∆G° changes to a value determined by the equilibrium constant; the v ...
Chp 5 Circle the correct answer Consider three 1
... a) Yes, ΔE = 0 at all times, which is why q = -w. b) No, ΔE does not always equal zero but this is only due to factors like friction and heat. c) No, ΔE does not always equal zero because it refers to the system’s internal energy which is affected by heat and work. d) No, ΔE never equals zero becaus ...
... a) Yes, ΔE = 0 at all times, which is why q = -w. b) No, ΔE does not always equal zero but this is only due to factors like friction and heat. c) No, ΔE does not always equal zero because it refers to the system’s internal energy which is affected by heat and work. d) No, ΔE never equals zero becaus ...
258-261
... n the last section we saw how to use the balanced equation for a reaction to calculate the numbers of moles of reactants and products for a particular case. However, moles represent numbers of molecules, and we cannot count molecules directly. In chemistry we count by weighing. Therefore, in this se ...
... n the last section we saw how to use the balanced equation for a reaction to calculate the numbers of moles of reactants and products for a particular case. However, moles represent numbers of molecules, and we cannot count molecules directly. In chemistry we count by weighing. Therefore, in this se ...
Chemical Equations and Reactions
... • If you fix everything except one element, and it is even on one side and odd on the other, double the first number, then move on from there. C4H10 + O2 CO2 + H2O ...
... • If you fix everything except one element, and it is even on one side and odd on the other, double the first number, then move on from there. C4H10 + O2 CO2 + H2O ...
Effect Of Convection For Gaseous Hydrochloride
... 100 000 t of the dust /year is produced. It cannot be recycled as a iron feedstock because it contains not only 50% of Fe but also 5-15% Zn, mostly in form of zinc ferrite (franklinite) ZnFe2O4, and further minor compounds (about 1%) of Pb, Cd etc. The steelmaking dust should be therefore dumped as ...
... 100 000 t of the dust /year is produced. It cannot be recycled as a iron feedstock because it contains not only 50% of Fe but also 5-15% Zn, mostly in form of zinc ferrite (franklinite) ZnFe2O4, and further minor compounds (about 1%) of Pb, Cd etc. The steelmaking dust should be therefore dumped as ...
chapter15-burno.1348..
... Significance of the Equilibrium Constant The significance of the equilibrium constant lies in the fact that for a chemical reaction taking place at a particular temperature T, the equilibrium constant (KC or Kp) has a particular numerical value. This means that no matter what the starting concentra ...
... Significance of the Equilibrium Constant The significance of the equilibrium constant lies in the fact that for a chemical reaction taking place at a particular temperature T, the equilibrium constant (KC or Kp) has a particular numerical value. This means that no matter what the starting concentra ...
1 - Cathedral High School
... All these intermolecular forces are weaker than covalent bonds. For substances of similar molar mass, hydrogen bonds are stronger than dipoledipole attractions which are stronger than van der Waals' forces. Van der Waals' forces arise from the electrostatic attraction between temporary induced dipol ...
... All these intermolecular forces are weaker than covalent bonds. For substances of similar molar mass, hydrogen bonds are stronger than dipoledipole attractions which are stronger than van der Waals' forces. Van der Waals' forces arise from the electrostatic attraction between temporary induced dipol ...
Learning objectives - The John Warner School
... fast rate and a slow rate. They then compare their findings with a small group of students, and they choose the best from their selection. Students could then make a class montage on sugar paper. You could support students by giving them some examples of fast and slow reactions that they could find ...
... fast rate and a slow rate. They then compare their findings with a small group of students, and they choose the best from their selection. Students could then make a class montage on sugar paper. You could support students by giving them some examples of fast and slow reactions that they could find ...
Matter and Measurement
... Formal Charges Formal charges are hypothetical charges calculated assuming that the electrons in a covalent bond are equally shared by both atoms. So in HCl, the formal charge on both H and Cl is zero ...
... Formal Charges Formal charges are hypothetical charges calculated assuming that the electrons in a covalent bond are equally shared by both atoms. So in HCl, the formal charge on both H and Cl is zero ...
How to Make a Collage
... Welcome to AP Chemistry! AP Chemistry is probably the most difficult AP course offered in high school. By signing up for this class, you are agreeing to (1) have a solid work ethic, (2) put in 1 – 2 hours per day on chemistry outside of the classroom, (3) have a secure working knowledge base of gene ...
... Welcome to AP Chemistry! AP Chemistry is probably the most difficult AP course offered in high school. By signing up for this class, you are agreeing to (1) have a solid work ethic, (2) put in 1 – 2 hours per day on chemistry outside of the classroom, (3) have a secure working knowledge base of gene ...
Chem BIG REVIEW - Jones-wiki
... A. Electrons absorb energy as they move to an excited state. B. Electrons release energy as they move to an excited state. C. Electrons absorb energy as they return to the ground state. D. Electrons release energy as they return to the ground state. 6. Which statement regarding red and green visible ...
... A. Electrons absorb energy as they move to an excited state. B. Electrons release energy as they move to an excited state. C. Electrons absorb energy as they return to the ground state. D. Electrons release energy as they return to the ground state. 6. Which statement regarding red and green visible ...
Experimental Study of Closed System in the Chlorine Dioxide
... When the mole ratio r is below or equal to 1.00 (see curves 1 and 2), the absorbance decreases along with the extension of reaction time at 350 nm and then does not change with the reaction time afterwards. Under the condition that r is greater than 1.00 (see curve 3 to curve 7), the absorbance incr ...
... When the mole ratio r is below or equal to 1.00 (see curves 1 and 2), the absorbance decreases along with the extension of reaction time at 350 nm and then does not change with the reaction time afterwards. Under the condition that r is greater than 1.00 (see curve 3 to curve 7), the absorbance incr ...
Teaching to Standards: Science
... have a chemical reaction. Say: Let’s review what we did. First we poured the liquid vinegar into the cups. Then we added some solutes—the flour to this cup, the salt to this cup, and the baking soda to this cup. We mixed the solutes with the vinegar and made different mixtures. And we watched to see ...
... have a chemical reaction. Say: Let’s review what we did. First we poured the liquid vinegar into the cups. Then we added some solutes—the flour to this cup, the salt to this cup, and the baking soda to this cup. We mixed the solutes with the vinegar and made different mixtures. And we watched to see ...
Battery Materials
... concentration of the vacant sites in the crystal lattice (together with the concentration of the redox species) ...
... concentration of the vacant sites in the crystal lattice (together with the concentration of the redox species) ...
FORMULA WRITNG
... (b) Write the formula for a covalent compound that can be formed from any two of the above five elements. __________ (c) Would you expect compound LZ to be a solid, liquid, or gas? An ionic or covalent substance? _________, ________ (d) Write a formula for a compound formed between elements M and X; ...
... (b) Write the formula for a covalent compound that can be formed from any two of the above five elements. __________ (c) Would you expect compound LZ to be a solid, liquid, or gas? An ionic or covalent substance? _________, ________ (d) Write a formula for a compound formed between elements M and X; ...
oxidation and reduction
... a) Oxidation and reduction are brought about by substances referred to as oxidising agents and reducing agents. Define each of these substances in terms of electrons. Oxidising agent ..................................................................................................................... ...
... a) Oxidation and reduction are brought about by substances referred to as oxidising agents and reducing agents. Define each of these substances in terms of electrons. Oxidising agent ..................................................................................................................... ...
Unit 4 - cloudfront.net
... 5. If the cell voltage is positive (_________________ cell), then the reaction is spontaneous, therefore the G= _____. If G is positive, then the reaction requires an input of energy to make it occur (_______________________ cell). If G = 0, then the reaction is at equilibrium. 6. Determine the ...
... 5. If the cell voltage is positive (_________________ cell), then the reaction is spontaneous, therefore the G= _____. If G is positive, then the reaction requires an input of energy to make it occur (_______________________ cell). If G = 0, then the reaction is at equilibrium. 6. Determine the ...
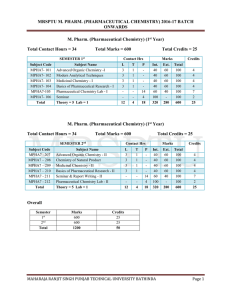
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
Chemical Reactions - Effingham County Schools
... Propane burns in excess oxygen according to the following reaction. ...
... Propane burns in excess oxygen according to the following reaction. ...
Chapter 7. CHEMICAL REACTIONS
... When solutions are involved in a reaction, only some of the ions present are usually involved. Other ions may be present, but they are still in the solution at the end of the reaction, unchanged by the chemical process. These ions are called spectator ions and are best left out of the balanced equat ...
... When solutions are involved in a reaction, only some of the ions present are usually involved. Other ions may be present, but they are still in the solution at the end of the reaction, unchanged by the chemical process. These ions are called spectator ions and are best left out of the balanced equat ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.