* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Types of Prints and Visualizing Prints
Bioorthogonal chemistry wikipedia , lookup
Multiferroics wikipedia , lookup
Thermal spraying wikipedia , lookup
Fluorescence wikipedia , lookup
Double layer forces wikipedia , lookup
Stoichiometry wikipedia , lookup
Protein adsorption wikipedia , lookup
Cosmic dust wikipedia , lookup
Photopolymer wikipedia , lookup
Click chemistry wikipedia , lookup
Daguerreotype wikipedia , lookup
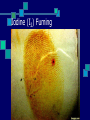
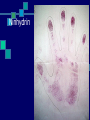
Types of Prints and Developing Prints Unit II Types of Prints: Plastic Molded or indented print Made from pressing a finger into a moldable material such as: Paint Putty Gum Stamps Softened candy bars Candle wax Types of Prints: Visible Left by a finger that touched a colored substance such as: Blood Dust Paint Mud Grease Chalk Types of Prints: Latent Invisible to the eye. Result from oil and water deposited from sweat pores on fingertips. Must be developed by chemical or physical means Developing Latent Prints There are several methods to develop latent prints. The method used depends on the surface you think the print might be on. Surfaces and Method Used Surface: Hard, non-absorbent surfaces such as glass, painted wood, tiles… Method: Dusting or Superglue Note: Choose a dusting powder that will stand out against the surface in question. Surfaces and Method Used Surface: Soft, porous surfaces such as Styrofoam, paper, leather… Method: Iodine, Ninhydrin, Silver Nitrate Note: The silver nitrate method is the same method used to develop pictures. Dusting Regular dust with a fiberglass brush Magnetic dust with a magnetic brush Fluorescent dust with a fiberglass brush Magnetic Fluorescent dust with a magnetic brush What is fluorescence? It occurs when a material absorbs light and then re-emits it at a longer wavelength than the light source. Usually use a UV (ultraviolet) light source. Can be high for low frequency UV Fluorescent Powder Iodine (I2) Fuming Iodine sublimes (solid to a gas) when heated. The material in question is placed in a closed container with I2 and heated. The I2 reacts with the oils in the print to make it visible. Once the process is stopped, the print will begin to fade. It must be photographed or sprayed with a 1% solution of starch in water, which will turn the print blue and make it last for several weeks to several months. Iodine (I2) Fuming Silver Nitrate (AgNO3) Silver nitrate reacts with the salt in your sweat. Reaction: AgNO3 + NaCl AgCl + NaNO3 Reasoning: The silver chloride (AgCl) produced is colorless, but when it is exposed to UV light, the print looks reddish-brown or black. Caution: be careful of skin exposure. Silver Nitrate (AgNO3) Ninhydrin This is a chemical reaction between the ninhydrin compound and the trace amounts of amino acids (building blocks of proteins) from your sweat. The reaction produces a purple-blue print. The reaction takes several hours, but is catalyzed (goes faster) with heat. Ninhydrin is sprayed on material. Caution: be careful of skin exposure. Ninhydrin Superglue Fuming Super Glue is approximately 98 to 99 percent cyanoacrylate. Cyanoacrylate fumes can be created when a Super Glue fuming chamber is made by putting the glue, the material, and a heat source in a tank. The fumes and the suspect material are contained within an enclosed chamber for up to 6 hours. Superglue Fuming