Section 1B
... After finding the masses of each element, these masses can be converted into moles. The ratio of these mole values gives the empirical formula. Example 1: To find ...
... After finding the masses of each element, these masses can be converted into moles. The ratio of these mole values gives the empirical formula. Example 1: To find ...
Unit 8 Test Review
... Use (g) for gaseous substances. Use (s) for solids. Use (l) for liquids. Use (aq) for species in solution in water. Write the state of matter immediately following the formula of the substance it describes. ...
... Use (g) for gaseous substances. Use (s) for solids. Use (l) for liquids. Use (aq) for species in solution in water. Write the state of matter immediately following the formula of the substance it describes. ...
Analytical animation-definitions electrochem3
... 2.1 Calculate the cell potential under standard condition of zinc-copper cell shown in model 1. ...
... 2.1 Calculate the cell potential under standard condition of zinc-copper cell shown in model 1. ...
Name_________________________________________
... Consider the following lab data used to find the density of an unknown solid sample Density Methanol = 0.791 g/ml Mass Flask + Stopper = 107.25 g Mass Flask + Stopper + Methanol = 154.47 g Mass Flask + Stopper + Solid = 132.42 g Mass Flask + Stopper + Solid+ Methanol = 176.12 g a) What is the volume ...
... Consider the following lab data used to find the density of an unknown solid sample Density Methanol = 0.791 g/ml Mass Flask + Stopper = 107.25 g Mass Flask + Stopper + Methanol = 154.47 g Mass Flask + Stopper + Solid = 132.42 g Mass Flask + Stopper + Solid+ Methanol = 176.12 g a) What is the volume ...
Chemical Equilibrium II
... Note that the equilibrium expression can be expressed by concentrations in terms of _________ for aqueous solutions or _________________ for gases (although for the purposes of Chemistry 12, we will not be using partial pressures) Some rules to follow when writing equilibrium expressions: “_________ ...
... Note that the equilibrium expression can be expressed by concentrations in terms of _________ for aqueous solutions or _________________ for gases (although for the purposes of Chemistry 12, we will not be using partial pressures) Some rules to follow when writing equilibrium expressions: “_________ ...
Characterization of thermal modulation of electrical conductivity: a
... is the solvent constant that is a function of the solvent density p, heat capacity C p , viscosity q , and the derivative of the viscosity with respect to temperature dq/dT. The solvent constant, Table 1, changes by about a factor of three for the different solvents listed (5,8-10). Interestingly, w ...
... is the solvent constant that is a function of the solvent density p, heat capacity C p , viscosity q , and the derivative of the viscosity with respect to temperature dq/dT. The solvent constant, Table 1, changes by about a factor of three for the different solvents listed (5,8-10). Interestingly, w ...
physical setting chemistry
... 68 Determine the total mass of 1-pentanol that will dissolve in 110. grams of water to produce a saturated solution. [1] ...
... 68 Determine the total mass of 1-pentanol that will dissolve in 110. grams of water to produce a saturated solution. [1] ...
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
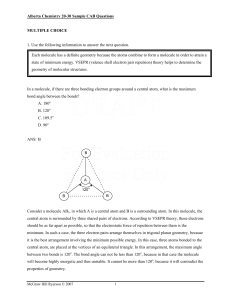
... Consider a molecule AB3, in which A is a central atom and B is a surrounding atom. In this molecule, the central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between ...
... Consider a molecule AB3, in which A is a central atom and B is a surrounding atom. In this molecule, the central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between ...
departmentofmaterials scienceandengineering
... reasons called an M-center) is the analogue of a He atom and comprises two electrons in a (somewhat extended and odd-shaped) box. Because one electron partially screens the positive charge for the second, the first excited electron state is lower in energy than that of the F-center, and the M-band a ...
... reasons called an M-center) is the analogue of a He atom and comprises two electrons in a (somewhat extended and odd-shaped) box. Because one electron partially screens the positive charge for the second, the first excited electron state is lower in energy than that of the F-center, and the M-band a ...
Chemistry COS 2011-2012
... Changes in environmental conditions can affect how beneficial a trait will be for the survival and reproductive success of an organism or an entire species. Throughout Earth’s history, extinction of a species has occurred when the environment changes and the individual organisms of that species do n ...
... Changes in environmental conditions can affect how beneficial a trait will be for the survival and reproductive success of an organism or an entire species. Throughout Earth’s history, extinction of a species has occurred when the environment changes and the individual organisms of that species do n ...
unit 4: chemical reaction rates
... Scientists discovered that by simply determining the mass of the substance, it was possible to count particles or atoms. A mole (mol) is the amount of a pure substance that contains the same amount of chemical units as there are atoms in exactly 12 grams of carbon, namely 12. In order to avoid confu ...
... Scientists discovered that by simply determining the mass of the substance, it was possible to count particles or atoms. A mole (mol) is the amount of a pure substance that contains the same amount of chemical units as there are atoms in exactly 12 grams of carbon, namely 12. In order to avoid confu ...
Answer
... What is the osmotic pressure of this solution at 37 C? The osmotic pressure for strong electrolyte solutions is given by: Π = i × (cRT) where i is the amount (mol) of particles in solution divided by the amount (mol) of dissolved solute. For 0.154 M NaCl, c = 0.154 and i = 2 (as NaCl Na+ + Cl-, e ...
... What is the osmotic pressure of this solution at 37 C? The osmotic pressure for strong electrolyte solutions is given by: Π = i × (cRT) where i is the amount (mol) of particles in solution divided by the amount (mol) of dissolved solute. For 0.154 M NaCl, c = 0.154 and i = 2 (as NaCl Na+ + Cl-, e ...
Chem152
... C) Brønsted-Lowry acid D) Brønsted-Lowry base E) none of the above 28. What is the term for the stage in an acid-base titration when the indicator changes color? A) color point B) endpoint C) indicator point D) stoichiometric point E) none of the above 29. What is the term for a chemical equation th ...
... C) Brønsted-Lowry acid D) Brønsted-Lowry base E) none of the above 28. What is the term for the stage in an acid-base titration when the indicator changes color? A) color point B) endpoint C) indicator point D) stoichiometric point E) none of the above 29. What is the term for a chemical equation th ...
Chemistry STUDY OF VOLUMETRIC AND VISCOMETRIC
... 0 reflects the volume change of the solute arising from the solute–solvent interactions. It means that the change in 0 at different [AcA] and temperature should reflect the changes occurring in its environment in the solution. The parameter Sv provides information regarding solute–solute interac ...
... 0 reflects the volume change of the solute arising from the solute–solvent interactions. It means that the change in 0 at different [AcA] and temperature should reflect the changes occurring in its environment in the solution. The parameter Sv provides information regarding solute–solute interac ...
What are the general types of reactions?
... – Magnesium and Hydrogen Chloride make Hydrogen and Magnesium Chloride – Ethylene (C2H4) burns with Oxygen to produce Carbon Dioxide and Water. – Hydrogen and Chlorine combine to make Hydrogen Chloride ...
... – Magnesium and Hydrogen Chloride make Hydrogen and Magnesium Chloride – Ethylene (C2H4) burns with Oxygen to produce Carbon Dioxide and Water. – Hydrogen and Chlorine combine to make Hydrogen Chloride ...
1994 Released Exam
... it. Select the one letteredchoice that bestanswerseach questionor bestfits each statementand then fill in the correspondingoval on the answersheet.A choice may be used once, more than once, or not at all in each set. Questions5-7 refer to the phasediagrambelow of a pure substance. ...
... it. Select the one letteredchoice that bestanswerseach questionor bestfits each statementand then fill in the correspondingoval on the answersheet.A choice may be used once, more than once, or not at all in each set. Questions5-7 refer to the phasediagrambelow of a pure substance. ...
Potassium Permanganate Solution
... After soaking, dry with an old soft towel. Please be aware this may become stained. For use in the bath Fill the bath as normal and then add a small amount of the purple liquid (Potassium Permanganate Solution) until the water turns “pale pink”. You will need to dissolve more than one tablet. One ta ...
... After soaking, dry with an old soft towel. Please be aware this may become stained. For use in the bath Fill the bath as normal and then add a small amount of the purple liquid (Potassium Permanganate Solution) until the water turns “pale pink”. You will need to dissolve more than one tablet. One ta ...
AP Chemistry (Zumdahl) Chapter 1 Notes: Chemical Foundations
... 1. Record all certain digits (all marked increments on a measuring tool) 2. Record the first digit that is uncertain, even if it is a zero. (All measurements have some degree of uncertainty.) 3. Uncertainty in the last number is + 1, unless otherwise noted B. Accuracy The agreement of a particular v ...
... 1. Record all certain digits (all marked increments on a measuring tool) 2. Record the first digit that is uncertain, even if it is a zero. (All measurements have some degree of uncertainty.) 3. Uncertainty in the last number is + 1, unless otherwise noted B. Accuracy The agreement of a particular v ...
CH100: Fundamentals for Chemistry
... Chemistry is the study of matter Matter is the “stuff” that makes up the universe The fundamental questions of Chemistry are: • How can matter be described? • How does one type of matter interact with other types of matter? • How does matter transform into other forms of matter? ...
... Chemistry is the study of matter Matter is the “stuff” that makes up the universe The fundamental questions of Chemistry are: • How can matter be described? • How does one type of matter interact with other types of matter? • How does matter transform into other forms of matter? ...
Chem 171-2-3: Final Exam Review Multiple Choice Problems 1
... Carbon disulfide is a liquid that can be used in the production of rayon and cellophane. It is manufactured from methane and elemental sulfur according to the following chemical equation: CH4 (g) + 4 S (s) Ÿ CS2 (l) + 2 H2S (g) How many moles of CS2 can be formed by the complete reaction of 10.6 mol ...
... Carbon disulfide is a liquid that can be used in the production of rayon and cellophane. It is manufactured from methane and elemental sulfur according to the following chemical equation: CH4 (g) + 4 S (s) Ÿ CS2 (l) + 2 H2S (g) How many moles of CS2 can be formed by the complete reaction of 10.6 mol ...
Публикация доступна для обсуждения в рамках
... In the simplified version the Eeqq − рН diagrams for the H2O−InAs and H2O−GaAs systems with description of typical reactions and stable forms of substances are presented in [15]. The similar diagram for indium antimonide is described in [3]. The values of Eeqq lie between the values of Eeq of the V ...
... In the simplified version the Eeqq − рН diagrams for the H2O−InAs and H2O−GaAs systems with description of typical reactions and stable forms of substances are presented in [15]. The similar diagram for indium antimonide is described in [3]. The values of Eeqq lie between the values of Eeq of the V ...
Introductary topics
... -ide becomes hydro-….-ic acid; H2S is hydrosulfuric acid -ate becomes -ic acid; H3PO4 is phosphoric acid -ite becomes -ous acid. HNO2 is nitrous acid ...
... -ide becomes hydro-….-ic acid; H2S is hydrosulfuric acid -ate becomes -ic acid; H3PO4 is phosphoric acid -ite becomes -ous acid. HNO2 is nitrous acid ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.