* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download AP Chemistry (Zumdahl) Chapter 1 Notes: Chemical Foundations
Dimensional analysis wikipedia , lookup
Atomic nucleus wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Analytical chemistry wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
AP Chemistry (Zumdahl)
Chapter 1 Notes: Chemical Foundations
1.1 Chemistry: An Overview
A. Reaction of hydrogen and oxygen
1. Two molecules of hydrogen react with one molecule of oxygen to form
two molecules of water: 2H2 + O2 → 2H2O
2. Decomposition of water: 2H2O → 2H2 + O2
B. Problem Solving in Chemistry (and life)
1. Making observations
2. Making a prediction
3. Do experiments to test the prediction
1.2 The Scientific Method
A. General Framework
1. Making observations
a. Quantitative ( measured)
b. Qualitative (not measured: color, phase, shape, ...)
2. Making a prediction
3. Do experiments to test the prediction
Results can become new observations, causing this to be a cycle.
B. Vocabulary
1. Observation
Something that is witnessed and can be recorded
2. Theory (Model)
a. Tested hypotheses that explains WHY nature behaves in a
certain way
b. Theories change as more information becomes available
3. Natural Law
A summary of observed, measurable behavior
1.3 Units of Measurement
A. Measurements
Number and Scale (units) are both essential.
A number without the units is worthless!
B. Fundamental SI Units
Mass:
kilogram
kg
Length:
meter
m
Time:
second
s
Temperature:
Kelvin
K
Amount of Substance:
mole
mol
Electric Current
ampere
A
Luminous Intensity
candela
cd
C. SI Prefixes
mega M 1,000,000 106
centi c 0.01
kilo k 1,000
103
milli m 0.001
1
deka D 10
10
micro μ 0.000001
deci d 0.1
10−1
nano n 0.000000001
10−2
10−3
10−6
10−9
1.4 Uncertainty in Measurement
A. Recording Measurements (Significant figures)
1. Record all certain digits (all marked increments on a measuring tool)
2. Record the first digit that is uncertain, even if it is a zero.
(All measurements have some degree of uncertainty.)
3. Uncertainty in the last number is + 1, unless otherwise noted
B. Accuracy
The agreement of a particular value with the accepted value
C. Precision
The degree of consistency among several measurements.
"You can be precise but inaccurate. But if you are accurate, you are
necessarily precise."
D. Errors
1. Random (indeterminate) Error
a. Measurements may be high or low
b. Usually caused by uncertainty in last digit of measurement
2. Systematic (determinate) Error
a. Always occur in the same direction
b. Caused by poor measurement calibration or technique
1) gun sight set too high/low
2) balance improperly zeroed
3) thermometer improperly marked
1.5 Significant Figures and Calculations
A. Rules for Counting Significant Figures
Number
Rule
Nonzero integers Always significant
Leading zeroes
Never significant
Captive zeroes
Always significant
Trailing zeroes
Insignificant if no decimal
Significant after a decimal
Scientific notation Coefficient is significant
Exact numbers
No impact on significance
Example
6.34 m
(3 sig figs)
0.00634 m (3 sig figs)
6.0034
(5 sig figs)
63400
(3 sig figs)
0.63400
(5 sig figs)
6.3400 x 106 (5 sig figs)
Counted items (30 stdnts)
Definitions (100 cm = 1 m)
B. Multiplication and Division
Keep as many sig. figs. in your answer as are in the measurement with
the fewest number of significant figures:
2.37 cm x 15.67 cm x 7.4 cm = 274.82046
keeping two sig. figs.: = 2.7 x 102 cm3
C. Addition and Subtraction
Compare the position of the last significant ("estimated") digit of each
measurement. The estimated digit in the leftmost column (tens,...tenths,
hundredths..) is the position of the estimated digit in the final answer.
34.039 m + 0.24 m + 1.332 m + 12.7 m = 48.311 m
(tenth's column is the largest uncertainty) = 48.3 m
D. Rules for Rounding
1. Round at the end of a series of calculations, NOT after each step
2. Look at the first digit to be eliminated when deciding whether to round.
If it is:
a. less than 5, the last significant digit is unchanged
b. 5 or more, the last significant digit is increased by 1
1.6 Dimensional Analysis
A. Conversion Factors have the effect of multiplying by one
Conversion values in the numerator are equivalent to denominator values.
B. Unit Conversions Questions
1. What units am I given?
2. What final units do I want in my answer?
3. What conversion factors do I use to get there?
C. Observe/include the following for all problems:
1. Significant figures
2. Correct units in all steps of your work
3. Correct units/label in your answer
4. Solve the problem in a manner that can be understood by the reader.
1.7 Temperature
A. Celsius (C) and Kelvin (K)
1. Kelvin = Celsius + 273.15
2. Celsius = Kelvin − 273.15
3. A change of 1C is the same as a change of 1K (∆C = ∆K)
B. Fahrenheit
1. TC = (TF − 32F)(5C/9F)
2. TF = TC x (9F/5C) + 32F
1.8 Density = mass/volume
1.9 Classification of Matter
A. Matter: Anything that occupies space and has mass
B. States of Matter
1. Solids - rigid, fixed volume and shape
2. Liquids - definite volume, no specific shape
3. Gases - no fixed volume or shape, highly compressible
C. Mixtures - Matter of variable composition
1. Heterogeneous mixtures have visibly distinguishable parts
2. Homogeneous mixtures (solutions) have visibly indistinguishable parts
3. Can be separated by physical means (that do not alter the identities)
a. Distillation
b. Filtration
c. Chromatography, ...
D. Pure substances have constant composition
1. Elements
Cannot be decomposed into simpler substances by physical nor
chemical means
2. Compounds
a. Constant composition
b. Can be broken into simpler substances by chemical means, not
by physical means
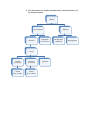
Matter
Pure Substances
Elements
Compounds
(bonded elements)
Atoms
Protons
(in nucleus)
Neutrons
(in nucleus)
Quarks
(2 up, 1 down)
Quarks
(1 up, 2 down)
Electrons
Mixtures
Homogeneous
(Solutions)
Heterogeneous