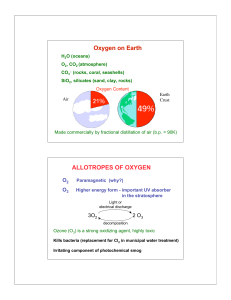
Solubility Equilibria
... MXn (s) Mn+ (aq) + nX‒ (aq) The equilibrium expression for the above solubility process is: Ksp = [Mn+][X‒]n Solubility product constant ...
... MXn (s) Mn+ (aq) + nX‒ (aq) The equilibrium expression for the above solubility process is: Ksp = [Mn+][X‒]n Solubility product constant ...
2 - Scheikundeolympiade
... contain an acidic hydrogen. C and E each have 3 other stereoisomers, while D and F each have 7 other ...
... contain an acidic hydrogen. C and E each have 3 other stereoisomers, while D and F each have 7 other ...
CHM – 124 Principles of Chemistry
... containingpolyatomic ions. Naming nonoxygen acids and ternary oxyacids. Naming inorganic bases. Section X - At the end of this section, the student should be able to: ...
... containingpolyatomic ions. Naming nonoxygen acids and ternary oxyacids. Naming inorganic bases. Section X - At the end of this section, the student should be able to: ...
Chapter 17, Section 17.3
... • Acid-base indicators: conjugate acidbase pairs ( HIn(aq)/In‾(aq) ) where acid and its conjugate base are different colours, for example: bromothymol blue HBb yellow/ Bb‾ blue ...
... • Acid-base indicators: conjugate acidbase pairs ( HIn(aq)/In‾(aq) ) where acid and its conjugate base are different colours, for example: bromothymol blue HBb yellow/ Bb‾ blue ...
EXPERIMENT 28 Intrinsic Viscosity: Chain Linkage in Polyvinyl
... quantitatively into a 250-mL volumetric flask. Avoid foaming as much as possible by letting the solution run down the side of the flask. Make the solution up to the mark with distilled water and mix by slowly inverting a few times. If the solution appears contaminated with insoluble material that w ...
... quantitatively into a 250-mL volumetric flask. Avoid foaming as much as possible by letting the solution run down the side of the flask. Make the solution up to the mark with distilled water and mix by slowly inverting a few times. If the solution appears contaminated with insoluble material that w ...
p-Block Elements, Part 1
... Silicate Minerals: [Si2O52-]n, SiO44Sand: SiO2 (this is also quartz). With aluminum in aluminosilicates (clay, feldspars). Prepared by: SiO2(s) + 2C(s) → Si(l) + 2CO(g) sand coke ...
... Silicate Minerals: [Si2O52-]n, SiO44Sand: SiO2 (this is also quartz). With aluminum in aluminosilicates (clay, feldspars). Prepared by: SiO2(s) + 2C(s) → Si(l) + 2CO(g) sand coke ...
1412_lecture_ch16 Fall_2014
... system will go in the forward or reverse direction requires that we evaluate the reaction quotient, Qc. If Qc exceeds the Ksp, precipitation occurs. If Qc is less than Ksp, more solute can dissolve. If Qc equals the Ksp, the solution is ...
... system will go in the forward or reverse direction requires that we evaluate the reaction quotient, Qc. If Qc exceeds the Ksp, precipitation occurs. If Qc is less than Ksp, more solute can dissolve. If Qc equals the Ksp, the solution is ...
The Major Classes of Chemical Reactions
... ions for each other. As a result, the ions become completely solvated, separate from one another, and move randomly throughout the solution. A similar scene occurs whenever an ionic compound dissolves in water. Although many ionic compounds dissolve in water, many others do not. In such cases, the e ...
... ions for each other. As a result, the ions become completely solvated, separate from one another, and move randomly throughout the solution. A similar scene occurs whenever an ionic compound dissolves in water. Although many ionic compounds dissolve in water, many others do not. In such cases, the e ...
CST Review Part 2
... Conservation of Matter and Stoichiometry State Standard #3 The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe ch ...
... Conservation of Matter and Stoichiometry State Standard #3 The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe ch ...
Practice Test 3: Answer Key
... A) All collisions of gaseous molecules are perfectly elastic. B) A mole of any gas occupies 22.4 L at STP. *** C) Gas molecules have no attraction for one another. D) The average kinetic energy for molecules is the same for all gases at the same temperature. ...
... A) All collisions of gaseous molecules are perfectly elastic. B) A mole of any gas occupies 22.4 L at STP. *** C) Gas molecules have no attraction for one another. D) The average kinetic energy for molecules is the same for all gases at the same temperature. ...
AH 2015 incl MG
... standard enthalpy of formation, ∆H f , in kJ mol−1, for Cu2+(Cl−)2(s). Using selected information from the thermochemical cycle above and the equation below calculate the standard enthalpy of formation, ∆H of , in kJ mol−1, of Cu+Cl−(s). Cu+(g) + Cl−(g) ...
... standard enthalpy of formation, ∆H f , in kJ mol−1, for Cu2+(Cl−)2(s). Using selected information from the thermochemical cycle above and the equation below calculate the standard enthalpy of formation, ∆H of , in kJ mol−1, of Cu+Cl−(s). Cu+(g) + Cl−(g) ...
PHYSICAL CHEMISTRY ERT 108 Semester II 2010
... Aims- find the equilibrium composition of an ideal-gas reaction mixture and - Equilibrium composition is related to the initial composition by a single variable, the equilibrium extent of reaction ...
... Aims- find the equilibrium composition of an ideal-gas reaction mixture and - Equilibrium composition is related to the initial composition by a single variable, the equilibrium extent of reaction ...
Chemistry booklet
... mℓ = 0, ONE s-orbital, mℓ = 0, ±1, THREE p-orbitals mℓ = 0, ±1, ±2, FIVE d-orbitals ms = SPIN quantum number, ms = +½ or -½. Eg. If n = 2, then ℓ = 0 or 1, and for ℓ = 0, mℓ = 0 ; and for ℓ = 1, mℓ = 0 +1 -1, For ℓ = 0, mℓ = 0 only : this defines one orbital – in this case the 2s-orbital. For ℓ = 1, ...
... mℓ = 0, ONE s-orbital, mℓ = 0, ±1, THREE p-orbitals mℓ = 0, ±1, ±2, FIVE d-orbitals ms = SPIN quantum number, ms = +½ or -½. Eg. If n = 2, then ℓ = 0 or 1, and for ℓ = 0, mℓ = 0 ; and for ℓ = 1, mℓ = 0 +1 -1, For ℓ = 0, mℓ = 0 only : this defines one orbital – in this case the 2s-orbital. For ℓ = 1, ...
2011
... Which of the following will cause an increase in the equilibrium concentration of CO? A) Increasing the pressure of the system at constant temperature. B) Adding more O2 to the system. C) Removing CO2 from the system as it is formed. D) Increasing the temperature of the system. ...
... Which of the following will cause an increase in the equilibrium concentration of CO? A) Increasing the pressure of the system at constant temperature. B) Adding more O2 to the system. C) Removing CO2 from the system as it is formed. D) Increasing the temperature of the system. ...
Chemistry Review 1 Answer Key
... the light with a spectroscope and observes four distinct spectral lines. The accompanying table gives the color, frequency, and energy for each of the four spectral lines. The unit for frequency is hertz, Hz. a.On the grid on the answer sheet or on a separate piece of paper, plot the data from the d ...
... the light with a spectroscope and observes four distinct spectral lines. The accompanying table gives the color, frequency, and energy for each of the four spectral lines. The unit for frequency is hertz, Hz. a.On the grid on the answer sheet or on a separate piece of paper, plot the data from the d ...
AP Chem -‐ Unit 1 Part 1 AP Chemistry 2016
... You are a public health officer in the water treatment facility for a city of 50,000 people. A concentration of 1.0 ppm of fluoride in the drinking water is sufficient for the purpose of helpi ...
... You are a public health officer in the water treatment facility for a city of 50,000 people. A concentration of 1.0 ppm of fluoride in the drinking water is sufficient for the purpose of helpi ...
Scientific Measurement
... A real gas will act most ideally under the conditions of low pressure and high temperature. (PLIGHT) ...
... A real gas will act most ideally under the conditions of low pressure and high temperature. (PLIGHT) ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.