Lecture 13 11-20-02
... Our strategy for sketching a titration curve is simple. We begin by drawing our axes, placing pH on the y-axis and volume of titrant on the x-axis. After calculating the volume of titrant needed to reach the equivalence point, we draw a vertical line that intersects the x-axis at this volume. Next, ...
... Our strategy for sketching a titration curve is simple. We begin by drawing our axes, placing pH on the y-axis and volume of titrant on the x-axis. After calculating the volume of titrant needed to reach the equivalence point, we draw a vertical line that intersects the x-axis at this volume. Next, ...
Foreign molecules and ions in beryl obtained by infrared and visible
... Beryl minerals of Serbia were slightly studied in the last century and despite that there is some obtainable data about main characteristics there is a limited amount of information about foreign molecules in the mineral structure. Two beryl samples from different locations in Serbia were examined i ...
... Beryl minerals of Serbia were slightly studied in the last century and despite that there is some obtainable data about main characteristics there is a limited amount of information about foreign molecules in the mineral structure. Two beryl samples from different locations in Serbia were examined i ...
Paper
... Examination of electron paramagnetic resonance (EPR) spectra of the isolated pairs of ions gives a valuable information about exchange interactions in the ground state. Interesting EPR spectra of Cu2+ pairs in CaO were observed in [1]. The spectra are typical for S = 1 in a tetragonal symmetry with ...
... Examination of electron paramagnetic resonance (EPR) spectra of the isolated pairs of ions gives a valuable information about exchange interactions in the ground state. Interesting EPR spectra of Cu2+ pairs in CaO were observed in [1]. The spectra are typical for S = 1 in a tetragonal symmetry with ...
Name: ______KEY__________________ Date: ______ CHM 130
... 14. (5 pts) An oven cleaning solution is 40.0 % by mass of NaOH. If one jar of this product contains 454 grams of solution, how many grams of NaOH does it contain? Show all work. Mass solute / mass solution x 100 = (x g / 454 g) x 100 = 40.0% x = 454 g * 0.400 = 181.6 g = 182 g ...
... 14. (5 pts) An oven cleaning solution is 40.0 % by mass of NaOH. If one jar of this product contains 454 grams of solution, how many grams of NaOH does it contain? Show all work. Mass solute / mass solution x 100 = (x g / 454 g) x 100 = 40.0% x = 454 g * 0.400 = 181.6 g = 182 g ...
Chapter 7
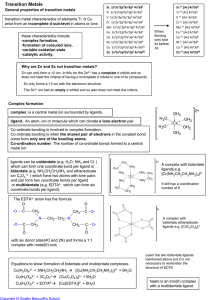
... the group numbers in the periodic table (the American and European numbering system) ...
... the group numbers in the periodic table (the American and European numbering system) ...
Topic 8-Solubility-Modified
... Their degree of ionization, their molecular size, the Interactions of substitute groups with solvent and their crystal properties. A} The influence of pH on aqueous solubility: 1} Acidic drugs: such as barbiturates, NSAID, nitrofurantoin, phenyl butazone, are less soluble in acidic solutions than in ...
... Their degree of ionization, their molecular size, the Interactions of substitute groups with solvent and their crystal properties. A} The influence of pH on aqueous solubility: 1} Acidic drugs: such as barbiturates, NSAID, nitrofurantoin, phenyl butazone, are less soluble in acidic solutions than in ...
Ministry of Education and Science of the Ukraine
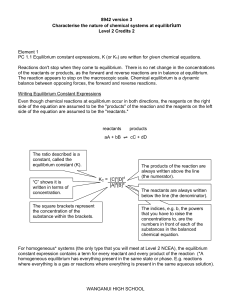
... the solution concentration: mass and volume percents, molar fraction, molarity, molality. Electrolytic dissociation, electrolytes. Strong and weak electrolytes. The degree of dissociation and dissociation constant. Ostwald’s dilution law. 5. Acid-base equilibrium in biological liquids. General prope ...
... the solution concentration: mass and volume percents, molar fraction, molarity, molality. Electrolytic dissociation, electrolytes. Strong and weak electrolytes. The degree of dissociation and dissociation constant. Ostwald’s dilution law. 5. Acid-base equilibrium in biological liquids. General prope ...
May 10th through May 14th
... • What are types of intermolecular forces? • What are dipole-dipole bonds? • Which is stronger Hydrogen or covalent bonds? ...
... • What are types of intermolecular forces? • What are dipole-dipole bonds? • Which is stronger Hydrogen or covalent bonds? ...
Unit 1 Notes (general chem review)
... To make a mirror for a telescope, you coat the glass with a thin layer of aluminum to reflect the light. If the mirror has a diameter of 6.0 inches, and you want to have a coating that is 0.015 mm thick, how many grams of aluminum will you need? How many atoms of aluminum are in the coating? The den ...
... To make a mirror for a telescope, you coat the glass with a thin layer of aluminum to reflect the light. If the mirror has a diameter of 6.0 inches, and you want to have a coating that is 0.015 mm thick, how many grams of aluminum will you need? How many atoms of aluminum are in the coating? The den ...
College Chemistry I PHS 1025 Fall 2012 Practice Exam 3A
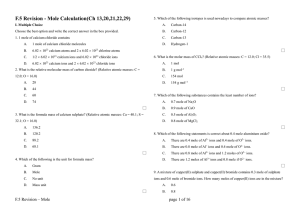
... 17) How many milliliters of 0.260 M Na2S are needed to react with 25.00 mL of 0.315 M AgNO3? Na2S(aq) + 2 AgNO3(aq) → 2 NaNO3(aq) + Ag2S(s) A) 60.6 mL B) 41.3 mL C) 15.1 mL ...
... 17) How many milliliters of 0.260 M Na2S are needed to react with 25.00 mL of 0.315 M AgNO3? Na2S(aq) + 2 AgNO3(aq) → 2 NaNO3(aq) + Ag2S(s) A) 60.6 mL B) 41.3 mL C) 15.1 mL ...
Oxidation-reduction reaction of chromium (VI) and iron (III) with
... The second order rate constants, k2 were determined as kobs/[PCM] and found to be 1.31 ± 0.12 dm3mol-1s-1and 1.16 ± 0.24dm3mol-1s-1for Cr (VI) and Fe (III) ions, respectively. The second order rate constants as seen in tables 2a and 2b above are fairly constant, further suggesting that the reactions ...
... The second order rate constants, k2 were determined as kobs/[PCM] and found to be 1.31 ± 0.12 dm3mol-1s-1and 1.16 ± 0.24dm3mol-1s-1for Cr (VI) and Fe (III) ions, respectively. The second order rate constants as seen in tables 2a and 2b above are fairly constant, further suggesting that the reactions ...
Practice Test Material - Directorate of Education
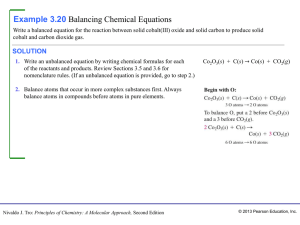
... Write the balanced ionic equation for the reaction of potassium dichromate with sodium sulphite to give Cr(III) and sulphate ions. ...
... Write the balanced ionic equation for the reaction of potassium dichromate with sodium sulphite to give Cr(III) and sulphate ions. ...
C. 3.5 g
... chloride in distilled water. What is the volume of the solution formed? (Relative atomic masses: K = 39.1, Cl = 35.5) ...
... chloride in distilled water. What is the volume of the solution formed? (Relative atomic masses: K = 39.1, Cl = 35.5) ...
введение в общую introductio to the general ch ведение в общую
... The question from the title of this subsection is very important. It can be rephrased in the following ways. What is chemistry? What is the subject of the discipline you are starting (or, hopefully, continuing) to study? What is the difference between chemistry and physics? Physical properties of a ...
... The question from the title of this subsection is very important. It can be rephrased in the following ways. What is chemistry? What is the subject of the discipline you are starting (or, hopefully, continuing) to study? What is the difference between chemistry and physics? Physical properties of a ...
PH

In chemistry, pH (/piːˈeɪtʃ/) is a numeric scale used to specify the acidity or alkalinity of an aqueous solution. It is the negative of the logarithm to base 10 of the activity of the hydrogen ion. Solutions with a pH less than 7 are acidic and solutions with a pH greater than 7 are alkaline or basic. Pure water is neutral, being neither an acid nor a base. Contrary to popular belief, the pH value can be less than 0 or greater than 14 for very strong acids and bases respectively.pH measurements are important in medicine, biology, chemistry, agriculture, forestry, food science, environmental science, oceanography, civil engineering, chemical engineering, nutrition, water treatment & water purification, and many other applications. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement.Primary pH standard values are determined using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode.The pH of aqueous solutions can be measured with a glass electrode and a pH meter, or indicator.pH is the negative of the logarithm to base 10 of the activity of the (solvated) hydronium ion, more often (albeit somewhat inaccurately) expressed as the measure of the hydronium ion concentration.The rest of this article uses the technically correct word ""base"" and its inflections in place of ""alkaline"", which specifically refers to a base dissolved in water, and its inflections.