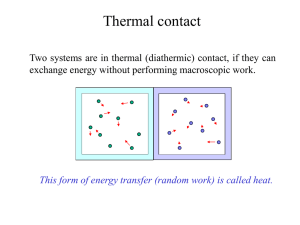
Lecture 14 Chapter 19 Ideal Gas Law and Kinetic Theory of Gases
... • They never will go back together even though energy of conservation is not violated. • Again what controls the direction? ...
... • They never will go back together even though energy of conservation is not violated. • Again what controls the direction? ...
thermodynamics and statistical physics
... spin of the particles. The temperature is such that kT = ". Calculate numerical values for the Fermi energy (use " as energy unit) and for the average number of particles in the lowest energy level if a) The three particles have spin 1/2. b) The three particles have spin 0. What causes the di erence ...
... spin of the particles. The temperature is such that kT = ". Calculate numerical values for the Fermi energy (use " as energy unit) and for the average number of particles in the lowest energy level if a) The three particles have spin 1/2. b) The three particles have spin 0. What causes the di erence ...
this contribution
... universe state with matter in thermal equilibrium. But thermal equilibrium is represented, in phase space P, as the coarse-graining sub-region of largest volume (so large that it normally exceeds all others put together). This corresponds to maximum entropy, so we reasonably ask: how can this be con ...
... universe state with matter in thermal equilibrium. But thermal equilibrium is represented, in phase space P, as the coarse-graining sub-region of largest volume (so large that it normally exceeds all others put together). This corresponds to maximum entropy, so we reasonably ask: how can this be con ...
Modeling and Analysis of Entropy Generation in Light
... Each of the three components (lattice, electrons and holes) is locally in thermodynamic equilibrium; (c) They are able to interchange energy with each other by various scattering mechanisms; (d) The three components are in thermal equilibrium; (e) The admissible states of electrons and holes are det ...
... Each of the three components (lattice, electrons and holes) is locally in thermodynamic equilibrium; (c) They are able to interchange energy with each other by various scattering mechanisms; (d) The three components are in thermal equilibrium; (e) The admissible states of electrons and holes are det ...
Thermodynamics
... brought into thermal contact, the spontaneous flow of heat that results is always from the high temperature object to the low temperature object. ...
... brought into thermal contact, the spontaneous flow of heat that results is always from the high temperature object to the low temperature object. ...
pdf 728k
... of a spontaneous process depends on how far the system was from its stable state. the more distant the system is from its equilibrium, the higher the spontaneity and quicker the rate of change towards equilibrium and the more permanent/irreversible the change will be. ...
... of a spontaneous process depends on how far the system was from its stable state. the more distant the system is from its equilibrium, the higher the spontaneity and quicker the rate of change towards equilibrium and the more permanent/irreversible the change will be. ...
First law
... Main article: Second law of thermodynamics The entropy of an isolated system consisting of two regions of space, isolated from one another, each in thermodynamic equilibrium in itself, but not in equilibrium with each other, will, when the isolation that separates the two regions is broken, so that ...
... Main article: Second law of thermodynamics The entropy of an isolated system consisting of two regions of space, isolated from one another, each in thermodynamic equilibrium in itself, but not in equilibrium with each other, will, when the isolation that separates the two regions is broken, so that ...
ch06A-2013
... ►Subtracting these equations: ►Since A and B are arbitrary internally reversible processes linking states 1 and 2, it follows that the value of the integral is independent of the particular internally reversible process and depends on the end states only. ...
... ►Subtracting these equations: ►Since A and B are arbitrary internally reversible processes linking states 1 and 2, it follows that the value of the integral is independent of the particular internally reversible process and depends on the end states only. ...
Solutions Exercises Lecture 2
... The entropy is a state function; thus the value of the change of the entropy is independent on the chosen path. If the real process is irreversible, then the reversible process from the same initial to the same end state needs to be defined for the computation of the change of entropy. The answer to ...
... The entropy is a state function; thus the value of the change of the entropy is independent on the chosen path. If the real process is irreversible, then the reversible process from the same initial to the same end state needs to be defined for the computation of the change of entropy. The answer to ...
Engineering Building Room 2303 Mail Code Phone: 818-677
... be true. If the system pressure equals the applied pressure no process will take place. We invoke the idealization known as a quasi-static process in which we assume that the changes in pressure are gradual so that the system pressure is essentially equal to the applied pressure at all times. This a ...
... be true. If the system pressure equals the applied pressure no process will take place. We invoke the idealization known as a quasi-static process in which we assume that the changes in pressure are gradual so that the system pressure is essentially equal to the applied pressure at all times. This a ...
Chapter 12
... If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy Degradation of energy is the conversion of high-grade energy to ...
... If form A can be completely converted to form B, but the reverse is never complete, A is a higher grade of energy than B When a high-grade energy is converted to internal energy, it can never be fully recovered as high-grade energy Degradation of energy is the conversion of high-grade energy to ...
Chapter 2 Classical Thermodynamics: The Second Law 2.1 Heat
... Also, from 1st law, dE = d̄Q + d̄W = d̄Qrev + d̄W rev = d̄Qirrev + d̄W irrev and, by definition of reversibility, for a given change of the system (e.g., given dV for a gas), d̄W irrev > d̄W rev (to overcome friction, etc.), we have d̄Qirrev < d̄Qrev , or d̄Q ≤ T dS ...
... Also, from 1st law, dE = d̄Q + d̄W = d̄Qrev + d̄W rev = d̄Qirrev + d̄W irrev and, by definition of reversibility, for a given change of the system (e.g., given dV for a gas), d̄W irrev > d̄W rev (to overcome friction, etc.), we have d̄Qirrev < d̄Qrev , or d̄Q ≤ T dS ...
Solutions Exercises Lecture 4
... B with a total mass of (mA + mB). The complete system is divided into two subsystems, namely A and B. Initially the two subsystems have different temperatures, namely TA and TB. At the end of the process the two systems have the same temperature; the colder systems has heated up while the initial wa ...
... B with a total mass of (mA + mB). The complete system is divided into two subsystems, namely A and B. Initially the two subsystems have different temperatures, namely TA and TB. At the end of the process the two systems have the same temperature; the colder systems has heated up while the initial wa ...
Slides
... is the electric susceptibility, and is the electric permittivity, or dielectric constant The field from a uniform dipole density is -4P, therefore the total field is ...
... is the electric susceptibility, and is the electric permittivity, or dielectric constant The field from a uniform dipole density is -4P, therefore the total field is ...
H-theorem

In classical statistical mechanics, the H-theorem, introduced by Ludwig Boltzmann in 1872, describes the tendency to increase in the quantity H (defined below) in a nearly-ideal gas of molecules. As this quantity H was meant to represent the entropy of thermodynamics, the H-theorem was an early demonstration of the power of statistical mechanics as it claimed to derive the second law of thermodynamics—a statement about fundamentally irreversible processes—from reversible microscopic mechanics.The H-theorem is a natural consequence of the kinetic equation derived by Boltzmann that has come to be known as Boltzmann's equation. The H-theorem has led to considerable discussion about its actual implications, with major themes being: What is entropy? In what sense does Boltzmann's quantity H correspond to the thermodynamic entropy? Are the assumptions (such as the Stosszahlansatz described below) behind Boltzmann's equation too strong? When are these assumptions violated?↑