Thermodynamics - myersparkphysics
... Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up hal ...
... Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up hal ...
Thermodynamics
... Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up hal ...
... Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up hal ...
The Second Law and the Concept of Entropy
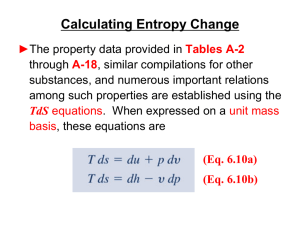
... No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work. Thus it is impossible to extract energy by heat from a high-temperature energy source and then convert all of the energy into work. At least some of the energy must be pa ...
... No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work. Thus it is impossible to extract energy by heat from a high-temperature energy source and then convert all of the energy into work. At least some of the energy must be pa ...
Absolute Rate Theory
... In this equation, k is the bimolecular rate constant for conversion of A and B to P and k' is the unimolecular rate constant for decomposition of the activated complex (AB)‡ to form P. Now, the Eyring approach assumes that we can assume a thermodynamic quasi-equilibrium to exist between A, B and (AB ...
... In this equation, k is the bimolecular rate constant for conversion of A and B to P and k' is the unimolecular rate constant for decomposition of the activated complex (AB)‡ to form P. Now, the Eyring approach assumes that we can assume a thermodynamic quasi-equilibrium to exist between A, B and (AB ...
AP Physics – Second Law of Thermodynamics
... The argument goes like this: the universe must go from an ordered state to a disordered state according to the second law of thermodynamics. Yet for life to have evolved as Darwin said it has would require that life have gone from a low state of order to a higher state of order. This is clearly proh ...
... The argument goes like this: the universe must go from an ordered state to a disordered state according to the second law of thermodynamics. Yet for life to have evolved as Darwin said it has would require that life have gone from a low state of order to a higher state of order. This is clearly proh ...
Chapter 19 Chemical Thermodynamics
... process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
... process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
Chapter 1. The Birth of Modern Physics
... The statistical interpretation of thermodynamics, through Statistical Mechanics (or Statistical Physics), was establish in the second half of the nineteenth century by Maxwell, Ludwig Eduard Boltzmann (1844-1906), and the American physicist J. Willard Gibbs (1839-1903). This theory bring us closer t ...
... The statistical interpretation of thermodynamics, through Statistical Mechanics (or Statistical Physics), was establish in the second half of the nineteenth century by Maxwell, Ludwig Eduard Boltzmann (1844-1906), and the American physicist J. Willard Gibbs (1839-1903). This theory bring us closer t ...
Fundamentals of Equilibrium Thermodynamics
... would involve an increase in entropy, and hence cannot be reversed under adiabatic conditions. To obtain the energy difference it suffices if the process can be carried out one way or the other. 4. It is possible to transform state A to B (or conversely) by other processes. The mechanical work wAB ...
... would involve an increase in entropy, and hence cannot be reversed under adiabatic conditions. To obtain the energy difference it suffices if the process can be carried out one way or the other. 4. It is possible to transform state A to B (or conversely) by other processes. The mechanical work wAB ...
ee10042808main.mov Example of Responding to an Unexpected
... (InqScribed by Corinne Manogue and Mary Bridget Kustusch) This narrative continues the examples of an instructor engaging students in thinking conceptually about the partial derivatives they encounter in thermodynamic contexts. In Name the Experiment sessions, the instructor, David Roundy, invites s ...
... (InqScribed by Corinne Manogue and Mary Bridget Kustusch) This narrative continues the examples of an instructor engaging students in thinking conceptually about the partial derivatives they encounter in thermodynamic contexts. In Name the Experiment sessions, the instructor, David Roundy, invites s ...
Carnot Cycle - University of Wyoming
... • A disorderly arrangement is much more probable than an orderly one if the laws of nature are allowed to act without interference – This comes from a statistical mechanics development ...
... • A disorderly arrangement is much more probable than an orderly one if the laws of nature are allowed to act without interference – This comes from a statistical mechanics development ...
H-theorem

In classical statistical mechanics, the H-theorem, introduced by Ludwig Boltzmann in 1872, describes the tendency to increase in the quantity H (defined below) in a nearly-ideal gas of molecules. As this quantity H was meant to represent the entropy of thermodynamics, the H-theorem was an early demonstration of the power of statistical mechanics as it claimed to derive the second law of thermodynamics—a statement about fundamentally irreversible processes—from reversible microscopic mechanics.The H-theorem is a natural consequence of the kinetic equation derived by Boltzmann that has come to be known as Boltzmann's equation. The H-theorem has led to considerable discussion about its actual implications, with major themes being: What is entropy? In what sense does Boltzmann's quantity H correspond to the thermodynamic entropy? Are the assumptions (such as the Stosszahlansatz described below) behind Boltzmann's equation too strong? When are these assumptions violated?↑