Chemistry 331 In Class Exercise Review for Final #1) (a) What are
... #24) The change in Enthalpy at constant pressure is equal to ___________ and the change in Internal Energy is equal to ___________ at constant volume. #25) If a chemical reaction releases heat into the environment it is call ______________ and if the chemical reaction absorbs heat from the environme ...
... #24) The change in Enthalpy at constant pressure is equal to ___________ and the change in Internal Energy is equal to ___________ at constant volume. #25) If a chemical reaction releases heat into the environment it is call ______________ and if the chemical reaction absorbs heat from the environme ...
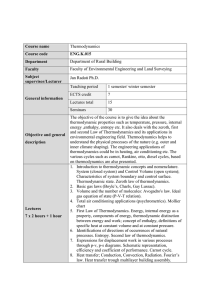
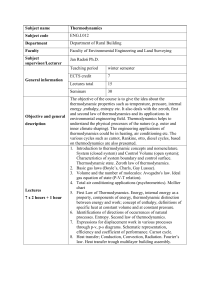
Basic thermodynamics` definitions. Units and conversions.
... and second Law of Thermodynamics and its applications in environmental engineering field. Thermodynamics helps to understand the physical processes of the nature (e.g. outer and inner climate shaping). The engineering applications of thermodynamics could be in heating, air conditioning etc. The vari ...
... and second Law of Thermodynamics and its applications in environmental engineering field. Thermodynamics helps to understand the physical processes of the nature (e.g. outer and inner climate shaping). The engineering applications of thermodynamics could be in heating, air conditioning etc. The vari ...
PY2P10: Thermodynamics Dr. Graham Cross www.tcd.ie/Physics/People/Graham.Cross
... not that system will change when brought into thermal contact with other systems. • Ie. Any two systems in equilibrium with the same temperature will also have to be in thermal equilibrium with each other. ...
... not that system will change when brought into thermal contact with other systems. • Ie. Any two systems in equilibrium with the same temperature will also have to be in thermal equilibrium with each other. ...
16 3.0 Chapter Contents 3.1 The Entropy and Internal Energy
... A functional relation between all extensive parameters of a thermodynamic system is called its fundamental equation (Gibbs, 1948). The fundamental equation contains all of the thermodynamic information on the system. Thermodynamic theory does not depend on the knowledge or even the existence of an e ...
... A functional relation between all extensive parameters of a thermodynamic system is called its fundamental equation (Gibbs, 1948). The fundamental equation contains all of the thermodynamic information on the system. Thermodynamic theory does not depend on the knowledge or even the existence of an e ...
BCJ0205-15 Thermal phenomena (3-1-4)
... After taking this course the student should have acquired knowledge, intuition and mathematical skills in physical situations involving: ...
... After taking this course the student should have acquired knowledge, intuition and mathematical skills in physical situations involving: ...
ENTROPY
... In the second place, and more important, no on knows what entropy really is, so in a debate you will always have the advantage.”” Note that compound probabilities are multiplicative, uncertainties are additive and so is entropy. For equally-probable microstates totalising a number Ω, their probabili ...
... In the second place, and more important, no on knows what entropy really is, so in a debate you will always have the advantage.”” Note that compound probabilities are multiplicative, uncertainties are additive and so is entropy. For equally-probable microstates totalising a number Ω, their probabili ...
Tutorial 3 – Thermodynamics of Dielectric Relaxations in Complex
... 1 - The local and instantaneous relations between thermal and mechanical properties of a physical system are the same as for a uniform system at equilibrium. This is the so-called local equilibrium hypothesis. ...
... 1 - The local and instantaneous relations between thermal and mechanical properties of a physical system are the same as for a uniform system at equilibrium. This is the so-called local equilibrium hypothesis. ...
8 Probability Distributions and Statistics
... identify k as Boltzmann’s constant and the Lagrange multiplier as 1 kT . We saw that k had to be Boltzmann’s constant to agree with thermodynamics. The identification of can be seen in two steps: (i) evaluate entropy S with the canonical distribution and (ii) demand that the result for dS is equ ...
... identify k as Boltzmann’s constant and the Lagrange multiplier as 1 kT . We saw that k had to be Boltzmann’s constant to agree with thermodynamics. The identification of can be seen in two steps: (i) evaluate entropy S with the canonical distribution and (ii) demand that the result for dS is equ ...
H-theorem

In classical statistical mechanics, the H-theorem, introduced by Ludwig Boltzmann in 1872, describes the tendency to increase in the quantity H (defined below) in a nearly-ideal gas of molecules. As this quantity H was meant to represent the entropy of thermodynamics, the H-theorem was an early demonstration of the power of statistical mechanics as it claimed to derive the second law of thermodynamics—a statement about fundamentally irreversible processes—from reversible microscopic mechanics.The H-theorem is a natural consequence of the kinetic equation derived by Boltzmann that has come to be known as Boltzmann's equation. The H-theorem has led to considerable discussion about its actual implications, with major themes being: What is entropy? In what sense does Boltzmann's quantity H correspond to the thermodynamic entropy? Are the assumptions (such as the Stosszahlansatz described below) behind Boltzmann's equation too strong? When are these assumptions violated?↑