
CHEM 240 Who am I?
... • Can we understand these changes? • Can we predict the transition temperature, i.e., the melting temperature Tm and the boiling temperature Tb, or the macroscopic properties of the different phases, e.g., the molar volumes, or the dependence of the transition temperatures on pressure on impurities ...
... • Can we understand these changes? • Can we predict the transition temperature, i.e., the melting temperature Tm and the boiling temperature Tb, or the macroscopic properties of the different phases, e.g., the molar volumes, or the dependence of the transition temperatures on pressure on impurities ...
Chapter 12: Thermodynamic Property Relations
... • The state postulate established that the state of a simple compressible system is completely specified by two independent, intensive properties. • Therefore, we should be able to calculate all the properties of a system such as internal energy, enthalpy, and entropy at any state once two independe ...
... • The state postulate established that the state of a simple compressible system is completely specified by two independent, intensive properties. • Therefore, we should be able to calculate all the properties of a system such as internal energy, enthalpy, and entropy at any state once two independe ...
The second law of thermodynamics
... We can therefore look at this combined system as two sub-systems (the original system and the heat bath) which are in contact. However, since the heat bath is much larger than our system, its temperature is not changed, because of its size. The temperature of the original system, when in equilibrium ...
... We can therefore look at this combined system as two sub-systems (the original system and the heat bath) which are in contact. However, since the heat bath is much larger than our system, its temperature is not changed, because of its size. The temperature of the original system, when in equilibrium ...
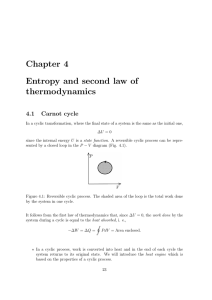
Chapter 4 Entropy and second law of thermodynamics
... multiplicity of = that correspond to the . a macrostate macrostate 2. The entropy can be defined in terms of the multiplicity as S ≡ kB ln (multiplicity) . We shall see in statistical mechanics that this definition is equivalent to the phenomenological concept we have learnt previously in this s ...
... multiplicity of = that correspond to the . a macrostate macrostate 2. The entropy can be defined in terms of the multiplicity as S ≡ kB ln (multiplicity) . We shall see in statistical mechanics that this definition is equivalent to the phenomenological concept we have learnt previously in this s ...
Chapter 19 Chemical Thermodynamics
... away from equil, sign of !G tells which way rxn goes Chemical ...
... away from equil, sign of !G tells which way rxn goes Chemical ...
Lecture 4
... numbers of molecules of the several chemical species present. If the conditions are changed slightly, but reversibly in such a way that the resulting system is also in equilibrium, we have ...
... numbers of molecules of the several chemical species present. If the conditions are changed slightly, but reversibly in such a way that the resulting system is also in equilibrium, we have ...
What is Thermodynamics?
... refrigeration, chemical reactions…); life sciences, with their complex molecular arrangements; nano–materials, where short range interactions are significant; complex fluids, like electrolytes and ionic fluids; critical behaviour and extraction processes (distillation…); search for new solvents; beh ...
... refrigeration, chemical reactions…); life sciences, with their complex molecular arrangements; nano–materials, where short range interactions are significant; complex fluids, like electrolytes and ionic fluids; critical behaviour and extraction processes (distillation…); search for new solvents; beh ...
Thermochemistry
... of water by one Celsius degree. (Food you eat is measured in Kilocalories which is abbreviated C). • Joule (J)-the SI unit of energy • 1 c=4.184J ...
... of water by one Celsius degree. (Food you eat is measured in Kilocalories which is abbreviated C). • Joule (J)-the SI unit of energy • 1 c=4.184J ...
Tue_10.00-Cadez
... Properties of non-neutral states • Charge density as a function of radius (for spherically symmetric boundary conditions!) The higher temperature component yields some entropy to allow the low temperature component to occupy more phase space, which makes the entropy of the complete system to increa ...
... Properties of non-neutral states • Charge density as a function of radius (for spherically symmetric boundary conditions!) The higher temperature component yields some entropy to allow the low temperature component to occupy more phase space, which makes the entropy of the complete system to increa ...
H-theorem

In classical statistical mechanics, the H-theorem, introduced by Ludwig Boltzmann in 1872, describes the tendency to increase in the quantity H (defined below) in a nearly-ideal gas of molecules. As this quantity H was meant to represent the entropy of thermodynamics, the H-theorem was an early demonstration of the power of statistical mechanics as it claimed to derive the second law of thermodynamics—a statement about fundamentally irreversible processes—from reversible microscopic mechanics.The H-theorem is a natural consequence of the kinetic equation derived by Boltzmann that has come to be known as Boltzmann's equation. The H-theorem has led to considerable discussion about its actual implications, with major themes being: What is entropy? In what sense does Boltzmann's quantity H correspond to the thermodynamic entropy? Are the assumptions (such as the Stosszahlansatz described below) behind Boltzmann's equation too strong? When are these assumptions violated?↑








![[cond-mat.stat-mech] 29 Jul 1999 - Data Analysis and Modeling of](http://s1.studyres.com/store/data/004609137_1-3d6203405239cf93abc08201b80fbc47-300x300.png)











