Chapter 6 Energy and Chemical Reactions
... Always have the same value whenever the system is in the same state. Two equal mass samples of water produced by: 1. Heating one from 20°C to 50°C. 2. Cooling the other from 100°C to 50°C. have identical final H (and V, P, E…). ...
... Always have the same value whenever the system is in the same state. Two equal mass samples of water produced by: 1. Heating one from 20°C to 50°C. 2. Cooling the other from 100°C to 50°C. have identical final H (and V, P, E…). ...
AP Physics Volume 2 Notes desktop
... or a tensile strength because it flows. **DO EXAMPLES ON PG. 261** ...
... or a tensile strength because it flows. **DO EXAMPLES ON PG. 261** ...
06_Lecture
... carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. Thermochemistry © 2012 Pearson Education, Inc. ...
... carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. Thermochemistry © 2012 Pearson Education, Inc. ...
chemical thermodynamics
... (a) Isothermic compression until 10 bar, followed by an isobaric heating until 300 °C (b) Isobaric heating until 300 °C, followed by the isothermic compression until 10 bar (c) Adiabatic compression, followed by heating or cooling until 300 °C. For each of this processes, calculate: i) Q, W e ∆U ii) ...
... (a) Isothermic compression until 10 bar, followed by an isobaric heating until 300 °C (b) Isobaric heating until 300 °C, followed by the isothermic compression until 10 bar (c) Adiabatic compression, followed by heating or cooling until 300 °C. For each of this processes, calculate: i) Q, W e ∆U ii) ...
First Law of Thermodynamics – Basic Concepts
... When a process goes from the initial to the final state in a single step and cannot be carried in the reverse order, it is said to be an irreversible process. Here the system is in equilibrium state in the beginning and at the end, but not at points in between. Consider a certain quantity of a gas c ...
... When a process goes from the initial to the final state in a single step and cannot be carried in the reverse order, it is said to be an irreversible process. Here the system is in equilibrium state in the beginning and at the end, but not at points in between. Consider a certain quantity of a gas c ...
thermochemistry
... out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. • qrxn = – Ccal × ∆T Thermochemistry © 2015 Pearson Education, Inc. ...
... out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. • qrxn = – Ccal × ∆T Thermochemistry © 2015 Pearson Education, Inc. ...
Chapter 5 Thermochemistry
... carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. Thermochemistry © 2012 Pearson Education, Inc. ...
... carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. Thermochemistry © 2012 Pearson Education, Inc. ...
Chapter 05 Notes
... carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. Thermochemistry © 2012 Pearson Education, Inc. ...
... carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. Thermochemistry © 2012 Pearson Education, Inc. ...
Ch04_Outline
... • After the sample (organic compound) has been placed in the bomb, the bomb is sealed and pressurized with oxygen. • It is then placed in the calorimeter, which is essentially an insulated container, and covered with an accurately measured quantity of water. • When all the components within the calo ...
... • After the sample (organic compound) has been placed in the bomb, the bomb is sealed and pressurized with oxygen. • It is then placed in the calorimeter, which is essentially an insulated container, and covered with an accurately measured quantity of water. • When all the components within the calo ...
Enthalpy, Entropy, Mollier Diagram and Steam
... realms, on certain complex topics, to facilitate comprehension of the relatively abstract thermodynamic concepts and principles. Each segment in this text concludes with a list of questions or problems, for self-assessment, skill building and knowledge affirmation purposes. The reader is encouraged ...
... realms, on certain complex topics, to facilitate comprehension of the relatively abstract thermodynamic concepts and principles. Each segment in this text concludes with a list of questions or problems, for self-assessment, skill building and knowledge affirmation purposes. The reader is encouraged ...
Biochemical Thermodynamics
... can be confident that all such changes—including the vast collection of physical and chemical changes we call life—must result only in the conversion of energy from one form to another or its transfer from place to place, not its creation or annihilation. ...
... can be confident that all such changes—including the vast collection of physical and chemical changes we call life—must result only in the conversion of energy from one form to another or its transfer from place to place, not its creation or annihilation. ...
Chapter 5 13edx
... out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. • qrxn = – Ccal × ∆T Thermochemistry © 2015 Pearson Education, Inc. ...
... out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. • qrxn = – Ccal × ∆T Thermochemistry © 2015 Pearson Education, Inc. ...
Journal of Alloys and Compounds Phase stability determination of
... Fig. 7 shows the assessed Mg–B phase diagrams at the external pressures of 1 MPa, 10 MPa and 100 MPa, respectively. When the external pressure is 1 MPa (Fig. 7(a)), Mg(l) phase exhibits a B solubility of 4.8% at 1465 ◦ C. The decomposition temperature of the MgB2 /MgB4 reaction increases from 1174 ◦ ...
... Fig. 7 shows the assessed Mg–B phase diagrams at the external pressures of 1 MPa, 10 MPa and 100 MPa, respectively. When the external pressure is 1 MPa (Fig. 7(a)), Mg(l) phase exhibits a B solubility of 4.8% at 1465 ◦ C. The decomposition temperature of the MgB2 /MgB4 reaction increases from 1174 ◦ ...
Chapter 5 Thermochemistry
... out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. • qrxn = – Ccal × ∆T Thermochemistry © 2015 Pearson Education, Inc. ...
... out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. • qrxn = – Ccal × ∆T Thermochemistry © 2015 Pearson Education, Inc. ...
Thermodynamics and Thermochemistry for Engineers
... A variable describing a particular piece of matter is said to be extensive if its value depends on the quantity of the matter being described. For example, total heat capacity and mass are both extensive variables as opposed to intensive variables such as density, specific heat capacity, and tempera ...
... A variable describing a particular piece of matter is said to be extensive if its value depends on the quantity of the matter being described. For example, total heat capacity and mass are both extensive variables as opposed to intensive variables such as density, specific heat capacity, and tempera ...
Basic Thermodynamics Prof. S. K. Som Department of Mechanical
... and our usual sign convention is the heat added to a system is positive while the work done by the system is positive. If you write it for an infinite small process with differential amount of the quantity, that means, if deltaQ is the infinite small heat added for an infinite small process executed ...
... and our usual sign convention is the heat added to a system is positive while the work done by the system is positive. If you write it for an infinite small process with differential amount of the quantity, that means, if deltaQ is the infinite small heat added for an infinite small process executed ...
Chapter 5: Thermochemistry
... temperature of the beverage to 37.0°C (normal body temperature). Can one lose weight by drinking ice-cold beverages if the body uses up about 1 calorie per gram of water per degree Celsius (i.e. the specific heat of water = 1.00 cal/g·°C) to consume the drink? a. Calculate the energy expended (in Ca ...
... temperature of the beverage to 37.0°C (normal body temperature). Can one lose weight by drinking ice-cold beverages if the body uses up about 1 calorie per gram of water per degree Celsius (i.e. the specific heat of water = 1.00 cal/g·°C) to consume the drink? a. Calculate the energy expended (in Ca ...
Chapter 2: Properties of Pure Substances We now turn our attention
... ♦ P-V-T Surface for a Substance that contracts upon freezing ...
... ♦ P-V-T Surface for a Substance that contracts upon freezing ...
Pressure
... Define A such that, when p equals some standard pressure, po, usually taken to be 1000 mb, T = . The quantity is called the potential temperature ...
... Define A such that, when p equals some standard pressure, po, usually taken to be 1000 mb, T = . The quantity is called the potential temperature ...
Thermochemistry Thermochemistry
... Enthalpy is a state function. Therefore we can can use some obvious properties to determine unknown enthalpies 1. Enthalpy changes are proportional to the amount of material 2. The sign of the enthalpy changes if a reaction is reversed 3. (Hess’s Law) If a reaction can be thought of as proceeding th ...
... Enthalpy is a state function. Therefore we can can use some obvious properties to determine unknown enthalpies 1. Enthalpy changes are proportional to the amount of material 2. The sign of the enthalpy changes if a reaction is reversed 3. (Hess’s Law) If a reaction can be thought of as proceeding th ...
Solutions Exercises Lecture 4
... change of enthalpy simplify for isobaric processes and the relationship between the enthalpy and the internal energy is well defined…. We start with the first law dU = dq + dw. The first law can be combined with the definition of the enthalpy H = U + p·V and with the total differential dH = dU + p·d ...
... change of enthalpy simplify for isobaric processes and the relationship between the enthalpy and the internal energy is well defined…. We start with the first law dU = dq + dw. The first law can be combined with the definition of the enthalpy H = U + p·V and with the total differential dH = dU + p·d ...
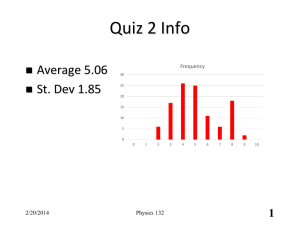
Physics 132 Prof. Buehrle 2/11/14
... same time its volume changes, but pressure is held constant. Which of the following is true? A. B. C. ...
... same time its volume changes, but pressure is held constant. Which of the following is true? A. B. C. ...
Document
... • We define _________________ as the amount of energy required to raise the temperature of 1 g of a substance by 1 ...
... • We define _________________ as the amount of energy required to raise the temperature of 1 g of a substance by 1 ...