* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Q4 U2 Energy and Chemical Reactions
International Energy Agency wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Energy storage wikipedia , lookup
Zero-energy building wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Alternative energy wikipedia , lookup
Negawatt power wikipedia , lookup
Compressed air energy storage wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Cogeneration wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Internal energy wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Conservation of energy wikipedia , lookup
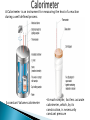
Q4U2 Law of Conservation of Energy: energy can not be created or destroyed, only changed from one form to another. Two Main Forms of Energy ◦ Kinetic energy is motion energy. Defined as the energy of a moving object. A thrown football, a speeding automobile, a waterfall, or a rock falling from a cliff are examples of objects that have kinetic energy. ◦ Potential energy is energy stored in matter. Potential energy appears in many different forms Defined as the energy in matter due to its position or the arrangement of its parts. •Rubber bands or Gasoline both have potential energy •The energy stored in molecules is called chemical potential energy. •This energy is stored in bonds •The chemical makeup (arrangement of molecules) of gasoline makes it a good fuel source (source of potential energy). •The energy stored in gasoline is released by burning it (combustion). •The airplane motor uses this released energy to turn a propeller. •During combustion, chemical bonds are broken and reformed creating new products and releasing energy. Is Related to heat lost or gained in chemical reactions Heat is released, given off, in an exothermic reaction Heat is absorbed, taken in, in an endothermic reaction The reaction between H2 and O2 is highly exothermic The energy from it powers cars The heat content of a chemical system is called the enthalpy (symbol: H) The enthalpy change (ΔH) is the amount of heat released or absorbed when a chemical reaction occurs at constant pressure. ΔH is specified per mole of substance Standard units are: ◦ kJ mol-1 (kJ/mol) or ◦ kcal mol-1 (kcal/mol) A calorie is the amount of heat needed to raise the temp of 1g of water by 1 ˚C 1 calorie (1 cal) = 4.184 joules (4.184 J) 1kilocalorie = 1 Calorie =1000 calories The Bond Enthalpy is the energy required to break a chemical bond. It is usually expressed in units of kJ mol-1, measured at 298 K. ◦ The Standard unit for energy is the joule Energy changes are measured under standard laboratory conditions (STP) Bond Enthalpy is the energy required to break a chemical bond. It is usually expressed in units of kJ/mol ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) Add up all the energies of the broken bonds; add up all the energies of the bonds that are reformed and subtract one from the other. 1. Find ΔH for the following reaction given the following bond energies: ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) Bond Bond Energy(kJ/mol) H-H 436 O=O 499 O-H 463 Figure out which bonds are broken and which bonds are formed. 2 H-H bonds are broken. 1 O=O bond is broken ◦ 2 O-H bonds are formed per water molecule, and there are 2 water molecules formed, therefore 4 O-H bonds are formed 2. Substitute the values given into the equation: Calculate the enthalpy of the combustion of Methane CH4 + O2 CO2 + H2O 1. Balance the equation CH4 + 2O2 CO2 + 2H2O 2. Write the Lewis Dot Structure H H-C-H, O=O, O=O O=C=O + H-O-H, H-O-H H 3. Count bonds broken and bonds Formed Bonds broken (reactants) Bonds Formed(Products) 4 C-H 413 kJ/mol each 2 C=O 799 kJ/mol each 2 O=O 495 kJ/mol each 4 O-H 463 kJ/mol each 4. 5. Calculate the sum of the bond enthalpies of products and reactants ∑ ΔH(bonds broken) = (4 X 413) + (2 X 495) = 2642 ∑ ΔH(bonds formed) = (2 X 799)+ ( 4 X 463) = 3450 Calculate the enthalpy of reaction ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) 2642 – 3450 = -808 kJ/mole Solutions 1. H-C-C-C-H + O=O O=C=O + H-O-H Broken bonds(ΔH each) Formed bonds(ΔH each) 8 C-H 413 kJ/mol 6 C=O 799 kJ/mol 2 C-C 348 kJ/mol 8 O-H 463 kJ/mol 5 O=O 495 kJ/mol ∑ΔH(bonds broken)=(8 X413)+(2X348)+(5X495)=6475 ∑ ΔH(bonds formed) = (6 X 799)+ ( 8 X 463) = 8498 ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) 6475 – 8498 = -2023 kJ/mole Solutions 2. H-C-C-O-H + O=O O=C=O + H-O-H Broken bonds(ΔH each) Formed bonds(ΔH each) 5 C-H 413 kJ/mol 4 C=O 799 kJ/mol 1 C-C 348 kJ/mol 6 O-H 463 kJ/mol 1 C-O 358 kJ/mol 1 O-H 463 kJ/mol 3 O=O 495 kJ/mol ∑ΔH(bonds broken)=(5 X413)+348+358+463+(3X495)=4719 kJ/mol ∑ ΔH(bonds formed) = (4 X 799)+ ( 6 X 463) = 5974 kJ/mol ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) 4719 – 5974 = -1255 kJ/mole Solutions H-C=C-H + O=O O=C=O + H-O-H Broken bonds(ΔH each) Formed bonds(ΔH each) 4 C-H 413 kJ/mol 8 C=O 799 kJ/mol 2 C=C 839 kJ/mol 4 O-H 463 kJ/mol 5 O=O 495 kJ/mol ∑ΔH(bonds broken)=(4 X413)+(2X 839)+(5X495)= 5805kJ/mol ∑ ΔH(bonds formed) = (8 X 799)+ ( 4 X 463) = 8744kJ/mol ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) 5805 – 8744 = - 2939kJ/mole N2 + 3H2 2NH3 Broken bonds(ΔH each) 1 N=N 941 kJ/mol 3 H-H 436 kJ/mol Formed bonds(ΔH each) 6 N-H 391 kJ/mol ∑ΔH(bonds broken)=941+(3X 436)= 2249kJ/mol ∑ ΔH(bonds formed) = (6 X 391) = 2346kJ/mol ΔH = ∑ ΔH(bonds broken) - ∑ ΔH(bonds formed) 2249– 2346 = -97 kJ/mole Exothermic Reaction Endothermic Reaction Energy is released. Energy is absorbed. Energy is a product of the reaction. Energy is a reactant of the reaction. Energy of the reactants is greater than the energy of the products ΔH(reactants) >ΔH(products) Energy of the reactants is less than the energy of the products ΔH(reactants) < ΔH(products) ΔH =ƸΔH(products) - ƸΔH(reactants) ΔH = Negative number ΔH = Positive number Heat of Reaction: The difference in energy between the Energy of the Products and the Energy of the Reactants. Δ H = H Prod - H Rxts •This is an exothermic reaction, the energy of the products is less than the energy of the reactants •The energy was lost to the environment as heat! •The Products have a higher energy level than reactants •Reactants are located on the flat portion to the left of the peak. •Products are located on the flat portion to the right of the peak. •The activated complex is located at the peak of the reaction diagram. An activated complex is an intermediate state that is formed during the conversion of reactants into products. It is the structure that results at the maximum energy point along the reaction path. The activation energy of a chemical reaction is the difference between the energy of the activated complex and the energy of the reactants. The lower the level of energy in a system the more stable it is(less likely to react). •The amount of energy required to start the reaction (Ea) •The difference between the energy level of the reactants and the maximum energy in the reaction progress equals the activation energy Catalysts lower the activation energy of a reaction allowing it to start more easily They do not effect the amount or type of product formed! Entropy is a measure of how much of the energy of a system is potentially available to do work and how much of it is potentially manifest as heat.(heat is often lost to the environment) Manufacturing equipment: heats the room because of heat loss to surroundings Example of ice melting the difference in temp. between a warm room (the surroundings) and a cold glass of ice water (the system) is equalized as heat from the warm surroundings are transferred to the cooler ice and water mixture. In an isolated system such as the room and ice water together, the dispersal of energy from warmer to cooler regions always results in a net increase in entropy The more disorder the higher the entropy! Complex (higher energy) (lower entropy) exothermic endothermic (higher entropy) Chaos (lower energy) A. B. C. D. E. F. Does the graph represent an endothermic or exothermic reaction? Label the position of the reactants, products, and activated complex. Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. Determine the activation energy, Ea for this reaction. How much energy is released or absorbed during the reaction? How much energy is required for this reaction to occur? A) The reaction is endothermic B) Reactants are located on the flat portion to the left of the peak. Products are located on the flat portion to the right of the peak. The activated complex is located at the peak of the reaction diagram. C) The enthalpy change is +50 kJ D) The activation energy is 200 kJ E) 50 kJ is absorbed in this reaction F) An input of 200 kJ is required for this reaction to occur Consider a general reversible reaction such as: A+B↔C+D Given the following potential energy diagram for this reaction, determine ΔH and Ea for both the forward and reverse directions. Is the forward reaction endothermic or exothermic? •Activation Energy (Ea) is the difference in energy between the activated complex and the reactants •Large energy barriers separates the reactants and the products so that only very energetic molecules can pass over this barrier. •The forward reaction is exothermic. When 2 moles of H2 react with 1 mole of O2 to produce 2 moles of H2O (g), heat is released. 2 H2 (g) + O2 (g) 2 H2O(g) + 484kj The heat needed to form a compound from its elements is called the energy of formation (∆Hf) ◦ This information is found in tables, for 1 mole of different compounds If the ∆Hf of each reactant and each product is known, a balanced equation can be used to calculate the energy released or absorbed during a reaction. The difference between the sum of the energies of the products and the sum of the energies of the reactants is the energy absorbed(+) or released(-) by the reaction ∆Hf(reaction) = ∑∆Hf(products) - ∑∆Hf(reactants) ∑ stands for the sum How much energy is produced by the reaction of 16.0g of Fe2O3 with excess aluminum metal according to the equation: Fe2O3(s) + 2Al(s) Al2O3(s) + 2Fe + 852 kj Steps to Solve 1. Begin with balanced equation above 2. Convert grams of Fe2O3(s) to moles 16.0g Fe2O3 1 mol Fe2O3 160g Fe2O3 3. From the equation, determine the number of moles and the energy involved. Calculate the answer 16.0g Fe2O3 1 mol Fe2O3 160g Fe2O3 852 kj = 85.2 kj produced 1 mol Fe2O3 1. A rocket fuel is prepared by reacting hydrazine and dinitrogen tetroxide according to the equation: 2N2H4 (l) + N2O4 (l) 3N2(g) +4H2O(g) + 2400 kj 2. calculate the heat released when 3200 grams of hydrazine are consumed in the rocket engine. The dissociation of ammonia into its elements is an endothermic reaction, absorbing 92.2kj of energy according to the equation: 2NH3(g) + 92.2 kj 3H2(g) + N2(g) How much energy will be required to decompose 85.0g of ammonia? 1. 2. 3200.0g N2H4 1 mol N2H4 1200 kj 32.0 g N2H4 1 mol N2H4 85.0gNH3 1 mol NH3 17.0 g NH3 = 120,000kj produced 46.1 kj = 231 kj produced 1 mol NH3 Chemistry Connections: Specific Heat Capacity and Calorimetry ◦ http://player.discoveryeducation.com/?blnPreviewO nly=1&guidAssetId=928367f4-9cf5-4ed6-bae5eded91b7dac9 The heat required to change the temperature of a substance depends upon the amount and nature of the substance as well as the extent of the temperature change For example: ◦ 1g of water requires 4.18joules of energy to change the temp1 ̊C. ◦ It takes only 0.987j to raise the temp of AlF3 1 ̊C Energy is transferred between a reaction and its surroundings. The amount transferred can be calculated: heat gained/lost= (mass)(change in temp)(specific heat) q=(m)(∆T)(Cp) q is heat gained/lost m is mass(g) ∆T is change in temp Cp is the specific heat A physical property of matter. The temperature of matter is a direct measure of the motion of the molecules ◦ The greater the motion the higher the temperature: Motion requires energy: The more energy matter has the higher temperature it will also have. ◦ Typically this energy is supplied by heat. ◦ Heat loss or gain by matter is equivalent to energy loss or gain How much will the temperature of an object increase or decrease by the gain or loss of heat energy? ◦ The answer is given by the specific heat (S) of the object. Heat is gained or lost during chemical and physical changes Calorimetry is a technique used to measure those changes in heat. Important definitions: ◦ Specific heat of water (Shw )=4.184 J / ( g oC ) This is the amount of heat required to raise the temp of 1 gram of water, 1 degree Celsius ◦ Heat of fusion of ice (Hfi )=334 J / g This is the amount of heat required to melt1 gram of ice at 0.00 degree Celsius (solid to liquid) ◦ Heat of vaporization of water (Hvw)=2256 J / g This is the amount of heat required to VAPORIZE 1 gram of water at 100.00 degrees Celsius (liquid to gas) How much heat is needed to melt a 60.0g ice cube at 0.0°C? ◦ Heat of fusion of ice (Hfi )=334 J / g ◦ Remember this is 1 gram of ice, we have 60.0g! ◦ 334 J g 60.0g = 20040 J 1 How much heat is needed to melt a 45.0g ice cube at 0.0°C? ◦ 334 J g 45.0g = 15030 J 1 How much heat is needed to vaporize 16.3g of water at 100.00 degrees Celsius? ◦ (Hvw)=2256 J / g ◦ 16.3 g 2256 J = 36772.8 J 1 g Take an object of mass m, put in x amount of heat and carefully note the temperature rise, then S is given by In this definition mass is usually in either grams or kilograms and temperature is either in kelvin or degrees Celsius. Note that the specific heat is "per unit mass". ◦ This means the volume does not matter 11 grams of substance is heated from 20.0˚C to 30.0˚C. The substance absorbed 4253 J of energy. What is the specific heat of the substance? S= X/(mass)(∆T) S = 4253J (11.0g)(10˚C) =38.6 J/g ˚C How much heat is required to raise the temperature of 68.0g of AlF3 from 25.0˚C to 80.0˚C ? Steps to Solve 1. Find specific heat of AlF3 from table Cp of AlF3 = 0.8948J/g ֹ C˚ 2. q=(m)(∆T)(Cp) q=(68.0g) (80.0 – 25.0˚C) (0.8948J/g ˚C) q= 3350 J A Calorimeter containing water is used to measure the heat absorbed or released in a chemical reaction. ◦ The temperature change in the water is used to measure the amount of heat absorbed or released by the reaction Law of conservation of energy says energy is neither created or destroyed. ◦ In an insulated system, any heat lost by one quantity of matter must be gained by another. Energy flows from the warmer material to the cooler material until they are both the same temperature. Heat lost = Heat gained m(∆T) Cp= m(∆T) Cp The heat evolved from the reaction changes the temperature of a working substance (usually water) with a known heat capacity. ◦ A measurement of the temperature rise in the surroundings (calorimeter body) allows a determination of the heat crossing the boundary between the system (where the reaction takes place) and the surroundings (where the temperature change is measured). A Calorimeter is an instrument for measuring the heat of a reaction during a well defined process A constant Volume calorimeter •A much simpler, but less accurate calorimeter, which, by its construction, is necessarily constant pressure Suppose a piece of lead with a mass of 14.9g at a temp of 92.5˚C is dropped into an insulated container of water. The mass of water is 165g and its temperature before adding the lead is 20.0˚C. What is the final temperature of the system? Cp lead =0.1276 J/g ֹ ˚C Remember Heat lost = Heat gained m(∆T) Cp= m(∆T) Cp (The lead will lose energy) a) The heat lost by lead is q= m(∆T) Cp = 14.9 g X (92.5 ˚C – Tf ) X 0.1276 J/gֹ ˚C b) The heat gained by water is q= m(∆T) Cp = 165g X (Tf - 20.0˚C) X 4.18 J/gֹ ˚C c) The heat gained must equal the heat lost: 165g X(Tf - 20.0˚C)X 4.18 J/gֹ ˚C=14.9 g X(92.5 ˚C– Tf )X 0.1276 J/gֹ ˚C (Tf - 20.0˚C) X 690. J/˚C = (92.5 ˚C – Tf ) X 1.90J/˚C (690. J/˚C )(Tf ) – 13800 J = 176 J – (1.90J/˚C )(Tf ) (690. J/˚C )(Tf ) + (1.90J/˚C )(Tf ) = 176 J + 13800 J (690. J/˚C + 1.90J/˚C )(Tf ) = 14000 J Tf = 20.2 ˚C 1. 2. 3. How much heat is absorbed by a reaction that lowers the temperature of 500.0g of water in a calorimeter by 1.10˚C ? Aluminum reacts with iron(III) oxide to yield aluminum oxide and iron. Calculate the heat given off in the reaction if the temperature of 1.00 kg of water in the calorimeter increases by 3.0˚C. Burning 1.00g of a fuel in a calorimeter raises the temperature of 1.0 kg of water from 20.0˚C to 28.05˚C. Calculate the heat given off in this reaction. How much heat would one mole of fuel give off, assuming the molar mass of 65.8g/mol? 1. qw = m(∆T) (Cw ) = 500.g(1.10˚C)(4.184J/g˚C) = 2300 J = 2.30 kJ 2. qw = m(∆T) (Cw ) = (1.0 x 103g)(3.00˚C)(4.184J/g˚C) = 12600 J = 12.6 Kj 3. ∆T = 28.05 ˚C – 20.0 ˚C = 8.05 ˚C for 1g: qw = m(∆T) (Cw ) = (1. 0 x 103g)(8.05˚C)(4.184J/g˚C) = 33700 J = 33.7 kJ for 1.0g for 1 mol: qw = (33.7 kj/1g)(65.8g/1mol) = 2220kJ/mol An equation which shows both mass and heat relationships between products and reactants is called a thermochemical equation. Example 2 H2(g) + O2(g) ----> 2 H2O(l) ΔH = -571.6 kJ The magnitude of ΔH is directly proportional to the amount of reactants or products. A + 2 B ----> C ΔH = -100 kJ 1/2 A + B ----> 1/2 C ΔH = -50 kJ ΔH for a reaction is equal in magnitude but opposite in sign for the reverse reaction. A + 2 B ----> C ΔH = -100 kJ C ----> A + 2 B ΔH = +100 kJ Hess' Law states that the heat evolved or constant heat summation. Hess's law can be applied to calculate enthalpies of reactions that are difficult to measure. absorbed in a chemical process is the same whether the process takes place in one or in several steps. This is also known as the law of For a chemical equation that can be written as the sum of two or more steps, the enthalpy change for the overall equation equals the sum of the enthalpy changes for the individual steps. ◦ No matter how you go from given reactants to products (whether in one step or several), the enthalpy change for the overall chemical change is the same. The heat transferred in a given change is the same whether the change takes place in a single step or in several steps. In order to use Hess's Law: 1) If a rxn is reversed, the sign of ΔH is reversed. S(s) + O2 (g) ---> SO2 (g) ΔH = -296 kJ SO2 (g) ---> S(s)+ O2 (g) ΔH = +296 kJ 2) If the coefficients in a balanced equation are multiplied by some number, the value of ΔH must be multiplied by that same number. S(s) + O2 (g) ---> SO2 (g) ΔH = -296 kJ 2 S(s) + 2O2 (g)--->2 SO2 (g) ΔH = (2)(-296 kJ) = -592 kJ Example S(s) + O2 (g) ---> SO2 (g) ΔH = -296 kJ SO2 (g) + 1/2 O2 (g) ---> SO3 (g) ΔH = -98.9 kJ ____________________________________________ S(s) + 1 1/2 O2 (g) ---> SO3 (g) ΔH = -394.9 kJ



























































![Second review [Compatibility Mode]](http://s1.studyres.com/store/data/003692853_1-a578e4717b0c8365c11d7e7f576654ae-150x150.png)
