Combustion Chemistry
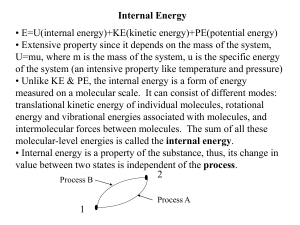
... • Internal energy is the total energy of molecules in the working fluid – a sum of kinetic and potential energies. ...
... • Internal energy is the total energy of molecules in the working fluid – a sum of kinetic and potential energies. ...
Welcome to Thermochemistry!
... to do work and is the sum of its enthalpy (H) plus the product of the temperature and the entropy (S) of the system. This quantity can be defined as: G=H−TS or more completely as G=U+PV−TS where •U = internal energy (SI unit: joule) •P = pressure (SI unit: pascal) •V = volume (SI unit: m 3 ) •T = te ...
... to do work and is the sum of its enthalpy (H) plus the product of the temperature and the entropy (S) of the system. This quantity can be defined as: G=H−TS or more completely as G=U+PV−TS where •U = internal energy (SI unit: joule) •P = pressure (SI unit: pascal) •V = volume (SI unit: m 3 ) •T = te ...
Chapter 8 Thermochemistry: Chemical Energy
... C(s) + O2(g) CO2(g) ΔHo = -393.5 kJ 2CO(g) + O2(g) 2CO2(g) Δ Ho = -566.0kJ 2H2 (g) + O2(g) 2H2O (g) Δ Ho = -483.6 kJ ...
... C(s) + O2(g) CO2(g) ΔHo = -393.5 kJ 2CO(g) + O2(g) 2CO2(g) Δ Ho = -566.0kJ 2H2 (g) + O2(g) 2H2O (g) Δ Ho = -483.6 kJ ...
Document
... The amount of heat required to raise the temperature of substance by 1C is known as molar heat capacity. cm =(C / n) (cm=molar heat capacity) ...
... The amount of heat required to raise the temperature of substance by 1C is known as molar heat capacity. cm =(C / n) (cm=molar heat capacity) ...
Superconcepts
... b. In thermochemistry, the system is the chemical reaction itself (only the reactants & products). The surroundings are everything else in the universe. c. First law of thermodynamics = energy is conserved, neither created nor destroyed; only transferred or transformed. d. Enthalpy = heat flow at co ...
... b. In thermochemistry, the system is the chemical reaction itself (only the reactants & products). The surroundings are everything else in the universe. c. First law of thermodynamics = energy is conserved, neither created nor destroyed; only transferred or transformed. d. Enthalpy = heat flow at co ...
Welcome to Thermochemistry!
... to do work and is the sum of its enthalpy (H) plus the product of the temperature and the entropy (S) of the system. This quantity can be defined as: G=H−TS or more completely as G=U+PV−TS where •U = internal energy (SI unit: joule) •P = pressure (SI unit: pascal) •V = volume (SI unit: m 3 ) •T = te ...
... to do work and is the sum of its enthalpy (H) plus the product of the temperature and the entropy (S) of the system. This quantity can be defined as: G=H−TS or more completely as G=U+PV−TS where •U = internal energy (SI unit: joule) •P = pressure (SI unit: pascal) •V = volume (SI unit: m 3 ) •T = te ...
thermodynamics - La Salle High School
... the most stable forms of elements assign “zero enthalpy” H ...
... the most stable forms of elements assign “zero enthalpy” H ...
thermochemistry - Pace University Webspace
... • Specific Heat is the amount of heat required to raise the temperature of one gram of the substance by one degree Celsius. Specific heat is an intensive property while heat capacity is an extensive property. ...
... • Specific Heat is the amount of heat required to raise the temperature of one gram of the substance by one degree Celsius. Specific heat is an intensive property while heat capacity is an extensive property. ...
Thermochemistry
... Second Law of Thermodynamics One statement defining the second law is that a spontaneous natural processes tend to even out the energy gradients in a isolated system. Can be quantified based on the entropy of the system, S, such that S is at a maximum when energy is most uniform. Can also be vi ...
... Second Law of Thermodynamics One statement defining the second law is that a spontaneous natural processes tend to even out the energy gradients in a isolated system. Can be quantified based on the entropy of the system, S, such that S is at a maximum when energy is most uniform. Can also be vi ...
Chemistry and the material world
... same final state, but now along a non-adiabatic way. That means that the system is in thermal contact with its surrounding and heat is allowed to flow. The energy difference ΔU will be the same (because it depends only on the state and not the way) but the work done doesn't necessarily have to be th ...
... same final state, but now along a non-adiabatic way. That means that the system is in thermal contact with its surrounding and heat is allowed to flow. The energy difference ΔU will be the same (because it depends only on the state and not the way) but the work done doesn't necessarily have to be th ...
FE Review Common Pitfalls in Thermodynamics
... 3. Algebra—Simple algebraic mistakes often occur in the solutions to thermodynamics problems. These mistakes unnecessarily lead to a great deal of student frustration that is not related to the thermodynamics theory applied to the problem solution. 4. System sketches—Students often omit sketches of ...
... 3. Algebra—Simple algebraic mistakes often occur in the solutions to thermodynamics problems. These mistakes unnecessarily lead to a great deal of student frustration that is not related to the thermodynamics theory applied to the problem solution. 4. System sketches—Students often omit sketches of ...
ET 11-08-14 SET 2
... 10. A single stage air turbine is to operate with air inlet pressure and temperature of 1 bar and 600 K. During the expansion the turbine losses are 20 kJ/kg to the surroundings which is at 1 bar and 300 K. For 1 kg of mass flow rate determine (i) decrease in availability (ii) Maximum work (iii) the ...
... 10. A single stage air turbine is to operate with air inlet pressure and temperature of 1 bar and 600 K. During the expansion the turbine losses are 20 kJ/kg to the surroundings which is at 1 bar and 300 K. For 1 kg of mass flow rate determine (i) decrease in availability (ii) Maximum work (iii) the ...
Chapter 12: Thermodynamic Property Relations
... completely specified by two independent, intensive properties. • Therefore, we should be able to calculate all the properties of a system such as internal energy, enthalpy, and entropy at any state once two independent, intensive properties are available. • The calculation of these properties from m ...
... completely specified by two independent, intensive properties. • Therefore, we should be able to calculate all the properties of a system such as internal energy, enthalpy, and entropy at any state once two independent, intensive properties are available. • The calculation of these properties from m ...
Brief 2-page Summary
... If a reaction is reversed, change the sign of its enthalpy but not the magnitude. If reactions are multiplied or divided by a fudge factor, multiply or divide the associated enthalpy. How? For each step and the final equation, find a unique chemical and determine whether the smaller reaction i ...
... If a reaction is reversed, change the sign of its enthalpy but not the magnitude. If reactions are multiplied or divided by a fudge factor, multiply or divide the associated enthalpy. How? For each step and the final equation, find a unique chemical and determine whether the smaller reaction i ...
PPF
... • It is also useful when one considers phase transition: Example: when liquid water vaporizes into water vapor, its internal energy changes from uf to ug. At the same time, itsa specific volume also changes from vf to vg, going through an expansion process; and it does work. Therefore, the total com ...
... • It is also useful when one considers phase transition: Example: when liquid water vaporizes into water vapor, its internal energy changes from uf to ug. At the same time, itsa specific volume also changes from vf to vg, going through an expansion process; and it does work. Therefore, the total com ...
chapter 5 thermochemistry
... functions; their values depend on the particular way in which a system changes its state. Sections 5.3 and 5.4 When a gas is produced or consumed in a chemical reaction occurring at constant pressure, the system may perform pressure-volume (P-V) work against the prevailing pressure. For this reason, ...
... functions; their values depend on the particular way in which a system changes its state. Sections 5.3 and 5.4 When a gas is produced or consumed in a chemical reaction occurring at constant pressure, the system may perform pressure-volume (P-V) work against the prevailing pressure. For this reason, ...
Solutions Exercises Lecture 2
... of 20 kg water of 200C. Work is done on the system by stirring so that the temperature in the system increases. The stirring is done with 0.25 kW or 0.25 kJ s-1. Or other, 0.25 kW = w/ t , with t indicating the time. To get an answer to the problem, first it needs to be determined how much work need ...
... of 20 kg water of 200C. Work is done on the system by stirring so that the temperature in the system increases. The stirring is done with 0.25 kW or 0.25 kJ s-1. Or other, 0.25 kW = w/ t , with t indicating the time. To get an answer to the problem, first it needs to be determined how much work need ...
Chapter 6:
... c. The equivalence of heat and work. d. The equivalence of internal energy and work. Answer: A 4. The statement, the internal work of an isolated system is constant, is also known as what? a. The first law of thermodynamics. b. The law of conservation of energy. c. The law of conservation of mass. d ...
... c. The equivalence of heat and work. d. The equivalence of internal energy and work. Answer: A 4. The statement, the internal work of an isolated system is constant, is also known as what? a. The first law of thermodynamics. b. The law of conservation of energy. c. The law of conservation of mass. d ...
HormonesCascade
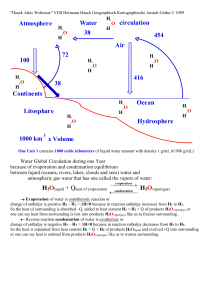
... One Unit 1 contains 1000 cubic kilometers of liquid water amount with density 1 g/mL (0.996 g/mL) ...
... One Unit 1 contains 1000 cubic kilometers of liquid water amount with density 1 g/mL (0.996 g/mL) ...
Text Questions
... 63. The greater the percentage of ________ and __________ in a fuel, the higher its fuel value. 64. Natural gas consists of _________ ______________. ...
... 63. The greater the percentage of ________ and __________ in a fuel, the higher its fuel value. 64. Natural gas consists of _________ ______________. ...
Thermodynamic Symbols and Constants
... The superscript o denotes the value given is for the standard reference state. For gases this is the ideal gas state at 1 atm. For example Hv refers to the enthalpy of vaporization of the liquid phase to the real gas at the saturation pressure, while Hov refers to the vaporization enthalpy of the ...
... The superscript o denotes the value given is for the standard reference state. For gases this is the ideal gas state at 1 atm. For example Hv refers to the enthalpy of vaporization of the liquid phase to the real gas at the saturation pressure, while Hov refers to the vaporization enthalpy of the ...