![[PDF]](http://s1.studyres.com/store/data/008813344_1-6b54197619c7ffbc0fcfc4fbb7e270fc-300x300.png)
[PDF]
... the temperature rises (or falls). The coupling also works in the opposite direction with the heat generation rate strongly depending on the electric field amplitude. Thus, the changes in the EM field distribution can increase or decrease the rate of temperature changes. The mathematical model that i ...
... the temperature rises (or falls). The coupling also works in the opposite direction with the heat generation rate strongly depending on the electric field amplitude. Thus, the changes in the EM field distribution can increase or decrease the rate of temperature changes. The mathematical model that i ...
Thermodynamics - cloudfront.net
... The Department of Energy (DOE) Fundamentals Handbooks consist of ten academic subjects, which include Mathematics; Classical Physics; Thermodynamics, Heat Transfer, and Fluid Flow; Instrumentation and Control; Electrical Science; Material Science; Mechanical Science; Chemistry; Engineering Symbology ...
... The Department of Energy (DOE) Fundamentals Handbooks consist of ten academic subjects, which include Mathematics; Classical Physics; Thermodynamics, Heat Transfer, and Fluid Flow; Instrumentation and Control; Electrical Science; Material Science; Mechanical Science; Chemistry; Engineering Symbology ...
Units and Properties - Instructor Guide - Final
... – Fluid under pressure has capacity to perform work • P-V energy (flow energy) foot-pounds force (ft-lbf) • Specific P-V energy of a substance is P-V energy per unit mass – Equals total P-V divided by total mass m, OR – Product of pressure P and specific volume v written as Pv o ft-lbf/lbm © Copyrig ...
... – Fluid under pressure has capacity to perform work • P-V energy (flow energy) foot-pounds force (ft-lbf) • Specific P-V energy of a substance is P-V energy per unit mass – Equals total P-V divided by total mass m, OR – Product of pressure P and specific volume v written as Pv o ft-lbf/lbm © Copyrig ...
Thermochemistry
... surroundings are that part of the universe outside the system with which the system interacts. Figure 7-1 pictures three common systems: first, as we see them and, then, in an abstract form that chemists commonly use. An open system freely exchanges energy and matter with its surroundings (Fig. 7-1a ...
... surroundings are that part of the universe outside the system with which the system interacts. Figure 7-1 pictures three common systems: first, as we see them and, then, in an abstract form that chemists commonly use. An open system freely exchanges energy and matter with its surroundings (Fig. 7-1a ...
Unit 10 Exam - Sharp Honors Chemistry Multiple Choice Identify the
... True or false? The law of conservation of energy states that energy can be converted from one form to another but can be neither created nor destroyed. a. True ...
... True or false? The law of conservation of energy states that energy can be converted from one form to another but can be neither created nor destroyed. a. True ...
CHAPTER 4: THERMODYNAMICS OF AIR
... what happens to a perfect gas in a control volume when mass leaves, mass enters, the volume changes, or heat is transferred. • Textbooks of thermodynamics rarely consider the first two scenarios, which are critical for our modelling work. Focus is on getting an expanding gas to do work by enlarging ...
... what happens to a perfect gas in a control volume when mass leaves, mass enters, the volume changes, or heat is transferred. • Textbooks of thermodynamics rarely consider the first two scenarios, which are critical for our modelling work. Focus is on getting an expanding gas to do work by enlarging ...
Lecture 18. The second law
... current intensity becomes zero; then the condenser discharge begins again through the coil and the process repeats itself with the current flowing in the opposite direction. In such a case we may use the second of Kirchhoff’s Laws for a closed circuit: the algebraic sum of all the e.m.f.’s and the d ...
... current intensity becomes zero; then the condenser discharge begins again through the coil and the process repeats itself with the current flowing in the opposite direction. In such a case we may use the second of Kirchhoff’s Laws for a closed circuit: the algebraic sum of all the e.m.f.’s and the d ...
chapter two internal energy and the first law of thermodynamics
... In the last chapter we briefly discussed ways in which a system can interact with its environment, and how this system can be characterized by an equation of state. This equation of state describes how the macroscopic parameters of the system are related to one another. As a simple, specific example ...
... In the last chapter we briefly discussed ways in which a system can interact with its environment, and how this system can be characterized by an equation of state. This equation of state describes how the macroscopic parameters of the system are related to one another. As a simple, specific example ...
Pdf - Text of NPTEL IIT Video Lectures
... not in a position that it will begin to vaporize. At state 2 is when you have saturated liquid that is 100 degree celsius, so water is in a position that it will begin to vaporize. State 2 corresponds to saturated liquid state. State 2 to state 3 is saturated mixture where you have liquid as well as ...
... not in a position that it will begin to vaporize. At state 2 is when you have saturated liquid that is 100 degree celsius, so water is in a position that it will begin to vaporize. State 2 corresponds to saturated liquid state. State 2 to state 3 is saturated mixture where you have liquid as well as ...
Documento PDF (pub54_99: 312 KB)
... processes and hence to state the second law of thermodynamics for cycles. In materials with memory, cycles are quite a few. The thermodynamic scheme is then completed by expressing the second law as a principle of dissipation of electromagnetic energy: the maximum work performed by the system in pas ...
... processes and hence to state the second law of thermodynamics for cycles. In materials with memory, cycles are quite a few. The thermodynamic scheme is then completed by expressing the second law as a principle of dissipation of electromagnetic energy: the maximum work performed by the system in pas ...
Chapter 6 - Educator
... Thus, work has the same unit as energy, the joule. To further illustrate the relationship between energy and work, let’s consider the bouncing tennis ball in Figure 6.2. First, we have to do work to raise the ball to its starting position. That is, we have to apply an upward force on the ball to ove ...
... Thus, work has the same unit as energy, the joule. To further illustrate the relationship between energy and work, let’s consider the bouncing tennis ball in Figure 6.2. First, we have to do work to raise the ball to its starting position. That is, we have to apply an upward force on the ball to ove ...
Chapter #10
... Energy is anything that has the capacity to do work. Work if force times distance Work units are Joules = kgm2/s2 Force is a push and has units of Newtons (kgm/s2) Although chemistry is the study of matter, matter is effected by energy. – It can cause physical and/or chemical changes in matter. ...
... Energy is anything that has the capacity to do work. Work if force times distance Work units are Joules = kgm2/s2 Force is a push and has units of Newtons (kgm/s2) Although chemistry is the study of matter, matter is effected by energy. – It can cause physical and/or chemical changes in matter. ...
Chapter 1 - Tarleton State University
... properties. The fourth unit used in the English system is the °F, which we use as an arbitrary measure of temperature. Temperature is also a thermodynamic property. The thermodynamic properties are then all derived, or defined, in terms of these four basic units. 1.1.2 Properties and State of a Subs ...
... properties. The fourth unit used in the English system is the °F, which we use as an arbitrary measure of temperature. Temperature is also a thermodynamic property. The thermodynamic properties are then all derived, or defined, in terms of these four basic units. 1.1.2 Properties and State of a Subs ...
Thermochemistry
... more abstract but nevertheless useful way, heat is the energy transferred between a system and its surroundings because of their difference in temperature. A combustion reaction, such as the burning of natural gas illustrated in Figure S.l(b), releases the chemical energy stored in the molecules of ...
... more abstract but nevertheless useful way, heat is the energy transferred between a system and its surroundings because of their difference in temperature. A combustion reaction, such as the burning of natural gas illustrated in Figure S.l(b), releases the chemical energy stored in the molecules of ...
Spring Book Problems - Blue Valley Schools
... Why do the strings used for the lowest-frequency notes on a piano normally have wire wrapped around them? What kind of waves do you think will travel down a horizontal metal rod if you strike its end (a) vertically from above and (b) horizontally parallel to its length? When a sinusoidal wave crosse ...
... Why do the strings used for the lowest-frequency notes on a piano normally have wire wrapped around them? What kind of waves do you think will travel down a horizontal metal rod if you strike its end (a) vertically from above and (b) horizontally parallel to its length? When a sinusoidal wave crosse ...
THERMOCHEMISTRY or Thermodynamics
... ENERGY is the capacity to do work or transfer heat. work is the energy used to cause an object with mass to move (w= F x d). When chemical reactions involve gases, the work done may involve compression or expansion of gases. (w = -P∆V where P = pressure and ∆V = Vfinal-Vinitial) ...
... ENERGY is the capacity to do work or transfer heat. work is the energy used to cause an object with mass to move (w= F x d). When chemical reactions involve gases, the work done may involve compression or expansion of gases. (w = -P∆V where P = pressure and ∆V = Vfinal-Vinitial) ...
H 2 (g) - SFP Online!
... ENERGY is the capacity to do work or transfer heat. work is the energy used to cause an object with mass to move (w= F x d). When chemical reactions involve gases, the work done may involve compression or expansion of gases. (w = -P∆V where P = pressure and ∆V = Vfinal-Vinitial) ...
... ENERGY is the capacity to do work or transfer heat. work is the energy used to cause an object with mass to move (w= F x d). When chemical reactions involve gases, the work done may involve compression or expansion of gases. (w = -P∆V where P = pressure and ∆V = Vfinal-Vinitial) ...
CHEM 155: BASIC PHYSICAL CHEMISTRY I INTRODUCTION
... Matter is any material substance that constitutes the observable universe and, together with energy, forms the basis of all objective phenomena. Depending on the temperature and other conditions, matter may appear in several states. The known states of matter are solid, liquid, gas, plasma and Bose- ...
... Matter is any material substance that constitutes the observable universe and, together with energy, forms the basis of all objective phenomena. Depending on the temperature and other conditions, matter may appear in several states. The known states of matter are solid, liquid, gas, plasma and Bose- ...
Energy
... – Work done on a system will increase the energy of the system, whereas work done by the system will decrease the energy of the ...
... – Work done on a system will increase the energy of the system, whereas work done by the system will decrease the energy of the ...
Vann - Chemistry ch. 6.1
... burning (usually with O2) in kJ/molrxn; note that combustion reactions yield oxides of that which is combusted • Enthalpy of formation (ΔHf) – heat absorbed or released when ONE mole of compound is formed from elements in their standard states in kJ/molrxn • Enthalpy of fusion (ΔHfus)—heat absorbe ...
... burning (usually with O2) in kJ/molrxn; note that combustion reactions yield oxides of that which is combusted • Enthalpy of formation (ΔHf) – heat absorbed or released when ONE mole of compound is formed from elements in their standard states in kJ/molrxn • Enthalpy of fusion (ΔHfus)—heat absorbe ...
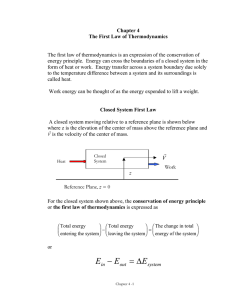
Chapter 4: The First Law of Thermodynamics
... Process 1-2c: Constant pressure These ideal gas processes have the same change in internal energy and enthalpy because the processes occur between the same temperature limits. ...
... Process 1-2c: Constant pressure These ideal gas processes have the same change in internal energy and enthalpy because the processes occur between the same temperature limits. ...
Chapter 10
... – Work done on a system will increase the energy of the system, whereas work done by the system will decrease the energy of the ...
... – Work done on a system will increase the energy of the system, whereas work done by the system will decrease the energy of the ...