Heriot-Watt University Scattering Dynamics of Oxygen Atoms on
... is to transform a Maxwell-Boltzmann distribution of translational energies at the surface temperature to a TOF distribution and adjust its amplitude to fit the slow (TD) component (red curves in Fig. 2). Then the IS component of the TOF is determined by subtracting the fitted TD component from the ...
... is to transform a Maxwell-Boltzmann distribution of translational energies at the surface temperature to a TOF distribution and adjust its amplitude to fit the slow (TD) component (red curves in Fig. 2). Then the IS component of the TOF is determined by subtracting the fitted TD component from the ...
Semester 2 review questions
... 3. Using the information from question (2) and the balanced equation, calculate the theoretical amount of Tin (Sn) that should have been produced from this reaction. (hint: determine the limiting reactant) ...
... 3. Using the information from question (2) and the balanced equation, calculate the theoretical amount of Tin (Sn) that should have been produced from this reaction. (hint: determine the limiting reactant) ...
Competitive Immunoassays for Simultaneous Detection of
... and the oxidative damage marker 3-nitrotyrosine (BSA-3NT) on a silicon nitride substrate. To demonstrate the versatility of the method, both direct and indirect format competitive immunoassays were developed and could be applied simultaneously for single samples. Signals from standard solutions were ...
... and the oxidative damage marker 3-nitrotyrosine (BSA-3NT) on a silicon nitride substrate. To demonstrate the versatility of the method, both direct and indirect format competitive immunoassays were developed and could be applied simultaneously for single samples. Signals from standard solutions were ...
Electrophilic Additions to Double Bonds
... electrons are too small and too light to be described by classical mechanics electrons need to be described by quantum mechanics accurate energy and potential energy surfaces for molecules can be calculated using modern electronic structure methods ...
... electrons are too small and too light to be described by classical mechanics electrons need to be described by quantum mechanics accurate energy and potential energy surfaces for molecules can be calculated using modern electronic structure methods ...
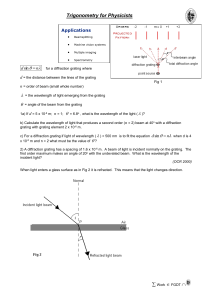
trigonometry
... b) If light arrives at an angle of 15o to the normal at an air-to-glass boundary for which the refractive index is 1.5, what is the expected angle of refraction? 4) The Refractive Index for an air-to-diamond boundary is about 2.42. If light arrives at an angle of 60o to the normal at an air-to-diamo ...
... b) If light arrives at an angle of 15o to the normal at an air-to-glass boundary for which the refractive index is 1.5, what is the expected angle of refraction? 4) The Refractive Index for an air-to-diamond boundary is about 2.42. If light arrives at an angle of 60o to the normal at an air-to-diamo ...
Unit 4 - Dorman High School
... Some polyatomic molecules can have dipole moments. When will this occur? IV. Stable Electron Configurations and Charges on Ions Look at the electron configuration of sodium: Look at the electron configuration of the sodium ion: Look at the electron configuration of neon: ...
... Some polyatomic molecules can have dipole moments. When will this occur? IV. Stable Electron Configurations and Charges on Ions Look at the electron configuration of sodium: Look at the electron configuration of the sodium ion: Look at the electron configuration of neon: ...
CHAPTER 1 Practice Exercises 1.1 x = 12.3 g Cd 1.3 2.24845 ×12 u
... A chemical reaction is a process whereby one or more chemical species is/are transformed into different chemical species. This generally involves the making and/or breaking of chemical ...
... A chemical reaction is a process whereby one or more chemical species is/are transformed into different chemical species. This generally involves the making and/or breaking of chemical ...
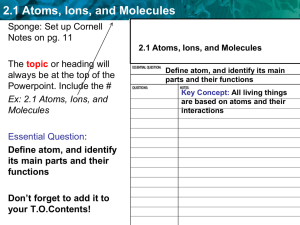
Atoms, Elements, Compounds File
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
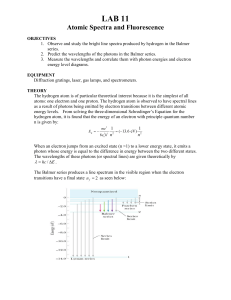
Lab 11: Atomic Spectra
... Part 1: The Atomic Spectrum of Fluorescent Lights a. Look at the fluorescent lights with the blue spectrometers. Write down the wavelengths of the most prominent lines you can see, along with their colors. (Some will be smeared so just do the best you can). b. Using the jpg image of the spectra of d ...
... Part 1: The Atomic Spectrum of Fluorescent Lights a. Look at the fluorescent lights with the blue spectrometers. Write down the wavelengths of the most prominent lines you can see, along with their colors. (Some will be smeared so just do the best you can). b. Using the jpg image of the spectra of d ...
word doc (perfect formatting)
... A. Rb-85 is approximately twice as abundant as Rb-87. B. Rb-85 is approximately three times as abundant as Rb-87. C. Rb-87 is approximately twice as abundant as Rb-85. D. Rb-87 is approximately three times as abundant as Rb-85 ...
... A. Rb-85 is approximately twice as abundant as Rb-87. B. Rb-85 is approximately three times as abundant as Rb-87. C. Rb-87 is approximately twice as abundant as Rb-85. D. Rb-87 is approximately three times as abundant as Rb-85 ...
biology biology - Napa Valley College
... A single covalent bond, or single bond, is the sharing of one pair of valence electrons A double covalent bond, or double bond, is the sharing of two pairs of valence electrons A triple covalent bond, or triple bond, is the sharing of three pairs of valence electrons ...
... A single covalent bond, or single bond, is the sharing of one pair of valence electrons A double covalent bond, or double bond, is the sharing of two pairs of valence electrons A triple covalent bond, or triple bond, is the sharing of three pairs of valence electrons ...
3 molecules
... MW of 164.2 g/mol and is 73.14 %C and 7.37 %H; the remainder is oxygen. What are the empirical and molecular ...
... MW of 164.2 g/mol and is 73.14 %C and 7.37 %H; the remainder is oxygen. What are the empirical and molecular ...
Building a Microwave Antenna for a Quantum Microscope
... When coupled, the pendulums will transfer energy. ...
... When coupled, the pendulums will transfer energy. ...
Lecture 24 (Slides) October 18
... molecules have many features in common. Individual atoms often possess unpaired electrons. These atoms are usually chemically unstable. Two such atoms can come together to form a molecule with no unpaired electrons. This process can involve the formation of covalent chemical bonds and is highly exot ...
... molecules have many features in common. Individual atoms often possess unpaired electrons. These atoms are usually chemically unstable. Two such atoms can come together to form a molecule with no unpaired electrons. This process can involve the formation of covalent chemical bonds and is highly exot ...
Document
... probability that the particle has momentum in the range (p, p+dp)? 14. Let s1 and s2 be the spin operators of two spin-1/2 particles. Find the simultaneous eigenfunctions of the operators s2 and sz, where s=s1+s2.show that these are also eigenfunctions of the operators s1 s2 . 15. Suppose an elec ...
... probability that the particle has momentum in the range (p, p+dp)? 14. Let s1 and s2 be the spin operators of two spin-1/2 particles. Find the simultaneous eigenfunctions of the operators s2 and sz, where s=s1+s2.show that these are also eigenfunctions of the operators s1 s2 . 15. Suppose an elec ...
Modeling excess heat and related issues Peter Hagelstein Research Laboratory of Electronics
... coherent energy exchange rate for a simplified version of the model in the strong coupling limit. The resulting rate can be fast as long as the coupling is sufficiently strong, and the scaling in the MeV to meV regime is gentle. ...
... coherent energy exchange rate for a simplified version of the model in the strong coupling limit. The resulting rate can be fast as long as the coupling is sufficiently strong, and the scaling in the MeV to meV regime is gentle. ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.