Investigation of the presence of rod-shaped bacteria on food surface
... length and outgoing secondary wavelength are identical, this method is called elastic scattering, while if there exists a wavelength shift, it is referred to as inelastic scattering. In the elastic scattering method, angular scatter measurements have been utilized for various quantitative studies of ...
... length and outgoing secondary wavelength are identical, this method is called elastic scattering, while if there exists a wavelength shift, it is referred to as inelastic scattering. In the elastic scattering method, angular scatter measurements have been utilized for various quantitative studies of ...
Which notation represents an atom of sodium
... suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson concluded that the atom was a positively charged sphere of almost uniform density in which negatively charged particles were embedded. The total negative charge in the atom was balanced by the posit ...
... suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson concluded that the atom was a positively charged sphere of almost uniform density in which negatively charged particles were embedded. The total negative charge in the atom was balanced by the posit ...
Comment on: Increasing Exclusion: The Pauli Exclusion
... Abstract: Phillips incorrectly analyzes the ionization of He to conclude that the Pauli principle and conservation of energy are mutually exclusive. His error arises from neglect of electron repulsion, improper choices of energy zeros and other trivial errors. Phillips has submitted four drafts of a ...
... Abstract: Phillips incorrectly analyzes the ionization of He to conclude that the Pauli principle and conservation of energy are mutually exclusive. His error arises from neglect of electron repulsion, improper choices of energy zeros and other trivial errors. Phillips has submitted four drafts of a ...
- Palisades School District
... compounds M(OH)2 and MCO3 , where M represents an unidentified metal. (a) Identify the charge of the M ion in the ionic compounds above. (b) At 25°C, a saturated solution of M(OH)2 has a pH of 9.15. Calculate the molar concentration of OH-(aq) in the saturated solution. 2. Zinc metal is added to a h ...
... compounds M(OH)2 and MCO3 , where M represents an unidentified metal. (a) Identify the charge of the M ion in the ionic compounds above. (b) At 25°C, a saturated solution of M(OH)2 has a pH of 9.15. Calculate the molar concentration of OH-(aq) in the saturated solution. 2. Zinc metal is added to a h ...
Discoveries: Atoms to Quarks
... nearly all electron negative-energy states are occupied and are not observable. electron transitions from a positive-energy to an occupied negative-energy state are forbidden by Pauli’s exclusion principle. electron transitions from a positive-energy state to an empty negative-energy state are ...
... nearly all electron negative-energy states are occupied and are not observable. electron transitions from a positive-energy to an occupied negative-energy state are forbidden by Pauli’s exclusion principle. electron transitions from a positive-energy state to an empty negative-energy state are ...
chemistry
... your answer sheet. Then and only then, place an X in ink in each penciled circle. Be sure to mark only one answer with an X in ink for each question. No credit will be given for any question with two or more X’s marked. The sample below indicates how your final choice should be marked with an X in i ...
... your answer sheet. Then and only then, place an X in ink in each penciled circle. Be sure to mark only one answer with an X in ink for each question. No credit will be given for any question with two or more X’s marked. The sample below indicates how your final choice should be marked with an X in i ...
QUANTUM NUMBERS
... these diagrams indicate which orbital energy levels are occupied by electrons for an atom or ion In fig.2 on p. 187, as atoms become larger & the main energy levels come closer, some sublevels may overlap Generally the sublevels for a particular value of n, increase in energy in the order of s ...
... these diagrams indicate which orbital energy levels are occupied by electrons for an atom or ion In fig.2 on p. 187, as atoms become larger & the main energy levels come closer, some sublevels may overlap Generally the sublevels for a particular value of n, increase in energy in the order of s ...
Snímek 1
... 1) Nuclei with value Z or N equal to 2, 8, 20, 28, 50, 82, 126 (magic numbers) are more stable (isotope occurrence, number of stable isotopes, behavior of separation energy magnitude). 2) Nuclei with magic Z and N have zero quadrupol electric moments zero deformation. 3) The biggest number of stab ...
... 1) Nuclei with value Z or N equal to 2, 8, 20, 28, 50, 82, 126 (magic numbers) are more stable (isotope occurrence, number of stable isotopes, behavior of separation energy magnitude). 2) Nuclei with magic Z and N have zero quadrupol electric moments zero deformation. 3) The biggest number of stab ...
Bohr`s Model of the Atom - Mr. Walsh`s AP Chemistry
... states” gave way to probability distributions, governed by Werner Heisenberg’s uncertainty principle, which states that there is a limit on how much certainty can exist in the state of a sub-atomic particle. For example, the more exactly an electron’s position is specified, the less the exactly the ...
... states” gave way to probability distributions, governed by Werner Heisenberg’s uncertainty principle, which states that there is a limit on how much certainty can exist in the state of a sub-atomic particle. For example, the more exactly an electron’s position is specified, the less the exactly the ...
Aalborg Universitet The Landauer-Büttiker formula and resonant quantum transport
... 2 Resonant Transport in a Quantum Dot In the previous section we have allowed the coupling constant τ (see (3)) to be arbitrarily large. The only assumption was that {−2tL, 2tL } was not in the point spectrum of K. We now look at the small coupling case, τ → 0. In this case we will assume that the s ...
... 2 Resonant Transport in a Quantum Dot In the previous section we have allowed the coupling constant τ (see (3)) to be arbitrarily large. The only assumption was that {−2tL, 2tL } was not in the point spectrum of K. We now look at the small coupling case, τ → 0. In this case we will assume that the s ...
Diffusing-wave spectroscopy in a shear flow
... that traverses a path of length s through the sample to the point where the light is detected. We note that the numerical factor of a applies to pure elongational flow, as well as to simple Couette flow, so long as the flow is two dimensional. This is because linear shear flow can always be decompos ...
... that traverses a path of length s through the sample to the point where the light is detected. We note that the numerical factor of a applies to pure elongational flow, as well as to simple Couette flow, so long as the flow is two dimensional. This is because linear shear flow can always be decompos ...
+ 2 HCL(aq) CaCl2(aq) + H2O(l) + CO2(g)
... Chemical Formula: States what elements a compound contains and the exact number of atoms of these elements. Oxidation Number: positive or negative number on the periodic table that indicates how many electrons an element has gained, lost or shared when bonding with another element. Polyatomic Atom: ...
... Chemical Formula: States what elements a compound contains and the exact number of atoms of these elements. Oxidation Number: positive or negative number on the periodic table that indicates how many electrons an element has gained, lost or shared when bonding with another element. Polyatomic Atom: ...
5 Electrons in Atoms
... Both of the known values in the problem are expressed with significant figures, so the answer must ...
... Both of the known values in the problem are expressed with significant figures, so the answer must ...
Free electrons
... neglect the interactions with other electrons and ions; no external electromagnetic fields - move uniformly in a straight line. In the presence of fields - move according to Newton's laws 2. Electrons move free only between collisions with scattering centers. Collisions, are instantaneous - abruptly ...
... neglect the interactions with other electrons and ions; no external electromagnetic fields - move uniformly in a straight line. In the presence of fields - move according to Newton's laws 2. Electrons move free only between collisions with scattering centers. Collisions, are instantaneous - abruptly ...
Nugget
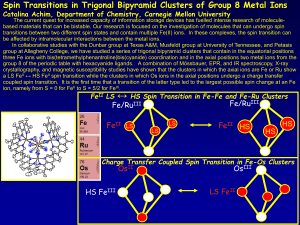
... The current quest for increased capacity of information storage devices has fuelled intense research of moleculebased materials that can be bistable. Our research is focused on the investigation of molecules that can undergo spin transitions between two different spin states and contain multiple Fe( ...
... The current quest for increased capacity of information storage devices has fuelled intense research of moleculebased materials that can be bistable. Our research is focused on the investigation of molecules that can undergo spin transitions between two different spin states and contain multiple Fe( ...
File
... Periods – elements are in order of their atomic number, each period has the same number of energy levels for electrons. Eg. Elements with 3 energy levels (more than 10 electrons) are found in period (row) 3. Groups – elements have similar properties as those found above and below them. Sometimes ...
... Periods – elements are in order of their atomic number, each period has the same number of energy levels for electrons. Eg. Elements with 3 energy levels (more than 10 electrons) are found in period (row) 3. Groups – elements have similar properties as those found above and below them. Sometimes ...
Environmental Physics for Freshman Geography Students Professor
... Planck’s constant is the underlying numerical constant behind so-called quantum mechanics; that is the mechanics that describes the way molecules, atoms and other small-scale systems interact with one another. One of the unfortunate aspects of modern physics is that there is no known simple way to p ...
... Planck’s constant is the underlying numerical constant behind so-called quantum mechanics; that is the mechanics that describes the way molecules, atoms and other small-scale systems interact with one another. One of the unfortunate aspects of modern physics is that there is no known simple way to p ...
ch6 - ChemistryVCE
... Both metallic and ionic lattices do contain positive ions in a regular arrangement. In a metallic lattice, the positive ions are surrounded by delocalised electrons; in an ionic lattice, negative ions alternate with the positive ions. Agree. In a metallic lattice, each positive ion attracts the delo ...
... Both metallic and ionic lattices do contain positive ions in a regular arrangement. In a metallic lattice, the positive ions are surrounded by delocalised electrons; in an ionic lattice, negative ions alternate with the positive ions. Agree. In a metallic lattice, each positive ion attracts the delo ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.