1/16/2015 Photoelectric Effect Part II The Einstein Equation
... Plug in the mercury lamp. It will take a few seconds for it to warm up. The window through which the light enters is covered by filters that only allow certain colors of light to pass. The filters are chosen to coincide with strong emission lines from mercury. The four wavelengths chosen are 390 nm, ...
... Plug in the mercury lamp. It will take a few seconds for it to warm up. The window through which the light enters is covered by filters that only allow certain colors of light to pass. The filters are chosen to coincide with strong emission lines from mercury. The four wavelengths chosen are 390 nm, ...
How many molecules?
... • Specific chemical composition of any mineral is a record of the melt or solution it precipitated from. Exact chemical composition of any mineral is a fingerprint, or a genetic record, much like your own DNA • This composition may be further affected by other processes • Can indicate provenance (or ...
... • Specific chemical composition of any mineral is a record of the melt or solution it precipitated from. Exact chemical composition of any mineral is a fingerprint, or a genetic record, much like your own DNA • This composition may be further affected by other processes • Can indicate provenance (or ...
New substances are formed by chemical reactions. When elements
... Take a look at this word equation for the reaction: copper + oxygen → copper oxide Copper and oxygen are the reactants because they are on the left of the arrow. Copper oxide is the product because it is on the right of the arrow. If we just replace the words shown above by the correct chemical form ...
... Take a look at this word equation for the reaction: copper + oxygen → copper oxide Copper and oxygen are the reactants because they are on the left of the arrow. Copper oxide is the product because it is on the right of the arrow. If we just replace the words shown above by the correct chemical form ...
Four Quantum Numbers
... S P D F: THE SUBLEVELS • Each of these 4 sublevels has a unique shape • Each orbital may contain at most, 2 electrons • LETTERS ORIGINATED FROM DESCRIPTIONS OF THEIR SPECTRAL LINES ...
... S P D F: THE SUBLEVELS • Each of these 4 sublevels has a unique shape • Each orbital may contain at most, 2 electrons • LETTERS ORIGINATED FROM DESCRIPTIONS OF THEIR SPECTRAL LINES ...
Physics Week 15(Sem. 2)
... quantum numbers, n, l ,ml , ms.” They can have any combination of numbers such that they aren’t identical, so the last number can be the only difference. ...
... quantum numbers, n, l ,ml , ms.” They can have any combination of numbers such that they aren’t identical, so the last number can be the only difference. ...
Alpha decay File
... at a distance b all the energy it expended in climbing to Vmax . The problem with α-decay is that Vmax is very large, and there is nowhere the α-particle could obtain so much energy within the nucleus. Where then does the energy come from? The quantum mechanical answer is that the particle does not ...
... at a distance b all the energy it expended in climbing to Vmax . The problem with α-decay is that Vmax is very large, and there is nowhere the α-particle could obtain so much energy within the nucleus. Where then does the energy come from? The quantum mechanical answer is that the particle does not ...
R E V I E W -- P R A C T I C E E X A
... e. All are TRUE 83. The tendency for an atom to attract the electrons to itself when it is combined with another atom: a. generally increases as you move from left to right from boron to fluorine b. generally increases as you move from right to left from phosphorous to aluminum c. eventually levels ...
... e. All are TRUE 83. The tendency for an atom to attract the electrons to itself when it is combined with another atom: a. generally increases as you move from left to right from boron to fluorine b. generally increases as you move from right to left from phosphorous to aluminum c. eventually levels ...
Photosynthesis Stores Energy in Organic Compounds
... concentration of H+ in thylakoid creates a concentration gradient. ...
... concentration of H+ in thylakoid creates a concentration gradient. ...
jeopardy review.
... Choose a category. You will be given the answer. You must give the correct question. Click to begin. ...
... Choose a category. You will be given the answer. You must give the correct question. Click to begin. ...
Praxis II Chemistry prep
... 1. Compare the atomic size of row 4 elements to row 5 elements. Describe the general trend as one goes from left to right across the periodic table. 1. Arrange the following ions in order of increasing size: Mg2+, Na+, Al3+ ...
... 1. Compare the atomic size of row 4 elements to row 5 elements. Describe the general trend as one goes from left to right across the periodic table. 1. Arrange the following ions in order of increasing size: Mg2+, Na+, Al3+ ...
June 2010 Regents Exam Part C Questions
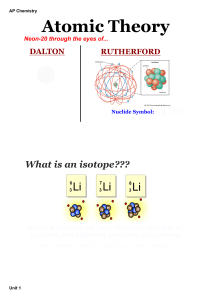
... Q 1-4 are based on Atom/Atomic Theory (items 1- 16 of the 200 Ways to Pass ….) Q1 The gold foil experiment led to the conclusion that each atom in the foil ...
... Q 1-4 are based on Atom/Atomic Theory (items 1- 16 of the 200 Ways to Pass ….) Q1 The gold foil experiment led to the conclusion that each atom in the foil ...
SAMPLE midterm with solutions
... 7. Explain why the quantum Hall effect is robust. The quantum Hall effect is robust because it exists so long as there are edge states at opposite sides of the sample, which carry current in one direction only and are in separate equilibrium. The states on a single edge are chiral, that is, they pro ...
... 7. Explain why the quantum Hall effect is robust. The quantum Hall effect is robust because it exists so long as there are edge states at opposite sides of the sample, which carry current in one direction only and are in separate equilibrium. The states on a single edge are chiral, that is, they pro ...
Using mass to calculate molecular formula
... Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number of atoms in the molecule. Percentages of ...
... Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number of atoms in the molecule. Percentages of ...
About UV-Vis Molecular Absorbance Spectroscopy
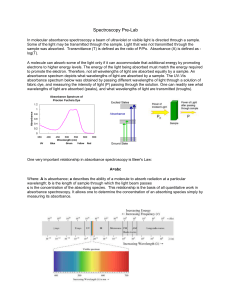
... sample was absorbed. Transmittance (T) is defined as the ratio of P/Po. Absorbance (A) is defined as log(T). A molecule can absorb some of the light only if it can accommodate that additional energy by promoting electrons to higher energy levels. The energy of the light being absorbed must match the ...
... sample was absorbed. Transmittance (T) is defined as the ratio of P/Po. Absorbance (A) is defined as log(T). A molecule can absorb some of the light only if it can accommodate that additional energy by promoting electrons to higher energy levels. The energy of the light being absorbed must match the ...
Chemistry Review Module Chapter 1
... Rule 5: Exponents and their bases, perfect multiples, uncertainties (error values), signs etc. are NEVER significant. 6.02 x 1023 has 3 significant digits 504.1 mL x 3 has 4 significant digits 5.3 ±0.5 mL has 2 significant digits –5.432 x 10-5 has 4 significant digits In each case, the blue part is ...
... Rule 5: Exponents and their bases, perfect multiples, uncertainties (error values), signs etc. are NEVER significant. 6.02 x 1023 has 3 significant digits 504.1 mL x 3 has 4 significant digits 5.3 ±0.5 mL has 2 significant digits –5.432 x 10-5 has 4 significant digits In each case, the blue part is ...
Photosynthesis Stores Energy in Organic Compounds
... concentration of H+ in thylakoid creates a concentration gradient. ...
... concentration of H+ in thylakoid creates a concentration gradient. ...
Modern Physics – Fall 2016 Prof. Akhavan Sharif University of
... In a muonic atom, the electron is replaced by a negatively charged particle called the muon. The muon mass is 207 times the electron mass. (a) What is the radius of the first Bohr orbit of a muonic lead(Z = 82) atom? (b) Calculate the magnitude of the lowest energy state for this atom. (c) Ignoring ...
... In a muonic atom, the electron is replaced by a negatively charged particle called the muon. The muon mass is 207 times the electron mass. (a) What is the radius of the first Bohr orbit of a muonic lead(Z = 82) atom? (b) Calculate the magnitude of the lowest energy state for this atom. (c) Ignoring ...
Unit chemical bonds
... Use element’s Valence electrons electrons in outer shell • Write elements chemical symbol • Use a dot to represent valence e-’s • Place valence e-’s around symbol ...
... Use element’s Valence electrons electrons in outer shell • Write elements chemical symbol • Use a dot to represent valence e-’s • Place valence e-’s around symbol ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.