topic 3: periodicity
... the increased nuclear charge; smaller atomic radius (outermost electron closer to nucleus); electrons go in the same energy level (similar shielding effect). The result is a stronger attraction which pulls the valence electrons closer to the nucleus/stronger ...
... the increased nuclear charge; smaller atomic radius (outermost electron closer to nucleus); electrons go in the same energy level (similar shielding effect). The result is a stronger attraction which pulls the valence electrons closer to the nucleus/stronger ...
Click here for printer-friendly sample test questions
... 12. When nuclear reactions are compared with ordinary chemical reactions, one MAJOR difference is A. the amount of energy released in nuclear reactions. B. the amount of matter absorbed in nuclear reactions. C. the loss of energy in chemical reactions. D. the gain of energy in chemical reactions. ...
... 12. When nuclear reactions are compared with ordinary chemical reactions, one MAJOR difference is A. the amount of energy released in nuclear reactions. B. the amount of matter absorbed in nuclear reactions. C. the loss of energy in chemical reactions. D. the gain of energy in chemical reactions. ...
WS-11-1
... greater lattice energy that cannot be overcome by the attraction of the water molecules. In NaOH the lattice energy is smaller than the favorable enthalpy of hydration, so it dissolves. 19. water : a, c and e CCl4 – b, d and f 20. Polarity, which creates hydrogen bonding and dipole. a. CH3CH2OH b. C ...
... greater lattice energy that cannot be overcome by the attraction of the water molecules. In NaOH the lattice energy is smaller than the favorable enthalpy of hydration, so it dissolves. 19. water : a, c and e CCl4 – b, d and f 20. Polarity, which creates hydrogen bonding and dipole. a. CH3CH2OH b. C ...
Electron Dynamics - CERN Accelerator School
... Adiabatic damping in linear accelerators In a linear accelerator: p⊥ ...
... Adiabatic damping in linear accelerators In a linear accelerator: p⊥ ...
Exam 1 Review Sheet Honors Biology This is to be used for
... you think we completely ignore gravity on the atomic level? (Hint: why do we ignore electrons when calculating mass?) 13. The nucleus of elements larger than hydrogen obviously has more than one proton in close proximity. How can this be if the electromagnetic force is pushing these like charges ap ...
... you think we completely ignore gravity on the atomic level? (Hint: why do we ignore electrons when calculating mass?) 13. The nucleus of elements larger than hydrogen obviously has more than one proton in close proximity. How can this be if the electromagnetic force is pushing these like charges ap ...
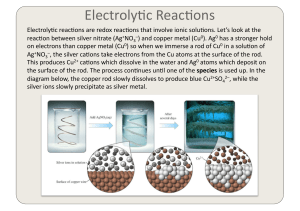
Electrochemistry 2
... Note the func)on of the salt bridge. If we have no salt bridge, the Cu half cell anode will start to lose electrons and generate Cu2+ ca)ons but it will not have enough nega)ve counter ions. ...
... Note the func)on of the salt bridge. If we have no salt bridge, the Cu half cell anode will start to lose electrons and generate Cu2+ ca)ons but it will not have enough nega)ve counter ions. ...
Master Equation Solver for Multi-Energy well Reactions
... to benzene formation and soot in flames. • In competition with 1CH2 + C2H2 → C2H2 + 3CH2 generating relatively unreactive 3CH2 ground state. • Harvey and Glowacki have implemented a routine in MESMER to account for ISC using non-adiabatic transition state theory. • Microcanonical rate coefficients, ...
... to benzene formation and soot in flames. • In competition with 1CH2 + C2H2 → C2H2 + 3CH2 generating relatively unreactive 3CH2 ground state. • Harvey and Glowacki have implemented a routine in MESMER to account for ISC using non-adiabatic transition state theory. • Microcanonical rate coefficients, ...
Notes on kinetic and potential energy
... Notes on kinetic and potential energy Dalton’s atomic theory says that atoms are not destroyed or created in chemical reactions, just rearranged. We can be even more explicit and say that the number of atoms, and the mass of each atom, are not changed in a chemical reaction. Thus there is overall co ...
... Notes on kinetic and potential energy Dalton’s atomic theory says that atoms are not destroyed or created in chemical reactions, just rearranged. We can be even more explicit and say that the number of atoms, and the mass of each atom, are not changed in a chemical reaction. Thus there is overall co ...
end of year review
... _____ 2. Which of the following did scientists learn about the atom from Rutherford’s gold foil experiment? A. Atoms combine in simple ratios to form compounds. B. Electrons travel around the nucleus of an atom in concentric circular paths. C. The mass of an atom and its positive charge are concentr ...
... _____ 2. Which of the following did scientists learn about the atom from Rutherford’s gold foil experiment? A. Atoms combine in simple ratios to form compounds. B. Electrons travel around the nucleus of an atom in concentric circular paths. C. The mass of an atom and its positive charge are concentr ...
An Overview of Computational Chemistry
... • the variational energy is an upper bound to the lowest energy of the system • any approximate wave function will yield an energy higher than the ground state energy • parameters in an approximate wave function can be varied to minimize the Evar • this yields a better estimate of the ground state e ...
... • the variational energy is an upper bound to the lowest energy of the system • any approximate wave function will yield an energy higher than the ground state energy • parameters in an approximate wave function can be varied to minimize the Evar • this yields a better estimate of the ground state e ...
1.1 to 1.4
... Molecular elements - whose molecules consist of more than one atom of the same element. Ex. H2(g), S8(s). ...
... Molecular elements - whose molecules consist of more than one atom of the same element. Ex. H2(g), S8(s). ...
Exam 2 Review - Iowa State University
... 1. Oxidation number of an element in its elementary or uncombined state is 0. 2. In an ionic compound, the oxidation number of a monatomic ion is the same as its charge. 3. Certain elements almost always have the same oxidation number. a. Group 1A elements = +1 b. Group 2A elements = +2 c. Group 3A ...
... 1. Oxidation number of an element in its elementary or uncombined state is 0. 2. In an ionic compound, the oxidation number of a monatomic ion is the same as its charge. 3. Certain elements almost always have the same oxidation number. a. Group 1A elements = +1 b. Group 2A elements = +2 c. Group 3A ...
Summer Resources - mvhs
... 4. Define atomic orbital according to the quantum mechanical model. >90% probability of finding an e¯ at any time. 5. a. What four letters are commonly used to identify the shapes of orbitals? s, p, d, f. c. What does the shape of an orbital tell us about the electron’s position? Where it is most li ...
... 4. Define atomic orbital according to the quantum mechanical model. >90% probability of finding an e¯ at any time. 5. a. What four letters are commonly used to identify the shapes of orbitals? s, p, d, f. c. What does the shape of an orbital tell us about the electron’s position? Where it is most li ...
How to deal with the loss in plasmonics and metamaterials
... of light in vacuum), corresponding to an operational wavelength of a few micrometres or less, most of the energy will be stored in the form of kinetic energy of electrons rather than as magnetic energy 6, which brings us back to the situation similar to that in Fig. 1c. Or, in other words, in struct ...
... of light in vacuum), corresponding to an operational wavelength of a few micrometres or less, most of the energy will be stored in the form of kinetic energy of electrons rather than as magnetic energy 6, which brings us back to the situation similar to that in Fig. 1c. Or, in other words, in struct ...
pptx
... what will you see from each atom? A. All atoms will emit the same colors. B. Atom 1 will emit more colors than 2 which will emit more colors than 3 C. Atom 3 will emit more colors than 2 which will emit more colors than 1 D. Atom 3 will emit more colors than 2. Atom 1 will emit no colors. E. Impossi ...
... what will you see from each atom? A. All atoms will emit the same colors. B. Atom 1 will emit more colors than 2 which will emit more colors than 3 C. Atom 3 will emit more colors than 2 which will emit more colors than 1 D. Atom 3 will emit more colors than 2. Atom 1 will emit no colors. E. Impossi ...
free electron theory
... Such a process which leads to the establishment of equilibrium in a system from which it was previously disturbed is called the relaxation process. The time taken for this process is RELAXATION TIME. ...
... Such a process which leads to the establishment of equilibrium in a system from which it was previously disturbed is called the relaxation process. The time taken for this process is RELAXATION TIME. ...
A solution to Maxwell`s equations in free space
... of 1km from the antenna, find (a) the amplitude of the electric and magnetic field strengths, and (b) the energy incident normally on a square plate of side 10cm in 5min. ...
... of 1km from the antenna, find (a) the amplitude of the electric and magnetic field strengths, and (b) the energy incident normally on a square plate of side 10cm in 5min. ...
7 - Mona Shores Blogs
... 35. Copper does not react with hydrochloric acid whereas manganese does? This means that a. copper is more active than hydrogen b. manganese is less active than hydrogen c. chloride ion will react with copper d. manganese is higher on the activity series than copper 36. What is the E for a system w ...
... 35. Copper does not react with hydrochloric acid whereas manganese does? This means that a. copper is more active than hydrogen b. manganese is less active than hydrogen c. chloride ion will react with copper d. manganese is higher on the activity series than copper 36. What is the E for a system w ...
Chapter 1 Chemistry: The Study of Matter
... can be broken down by chemical methods. When compounds are broken down, the components have completely different properties than the compound. ...
... can be broken down by chemical methods. When compounds are broken down, the components have completely different properties than the compound. ...
Atomic Systems and Bonding
... thus are not too tightly bound (making it easier to ‘move out’) outside shell had only one electron When the valence electron in any atom gains sufficient energy from some outside force, it can break away from the parent atom and become what is called a free electron Atoms with few electrons in thei ...
... thus are not too tightly bound (making it easier to ‘move out’) outside shell had only one electron When the valence electron in any atom gains sufficient energy from some outside force, it can break away from the parent atom and become what is called a free electron Atoms with few electrons in thei ...
Experimental basis for special relativity
... • According to Fresnel, the ether was dragged along with the earth and this gave rise to the aberration effect • However, Einstein gave the correct explanation in terms of relativistic velocity addition. A light ray will have a different angle in different relativistic frames of reference ...
... • According to Fresnel, the ether was dragged along with the earth and this gave rise to the aberration effect • However, Einstein gave the correct explanation in terms of relativistic velocity addition. A light ray will have a different angle in different relativistic frames of reference ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.