Atoms, Bonding, and the Periodic Table Understanding Main Ideas
... If the statement is true, write true. If the statement is false, change the underlined word or words to make the statement true. 1. _____________ An atom’s valence electrons are those electrons that have the highest energy. 2. _____________ Atoms tend to be stable and nonreactive if they have six va ...
... If the statement is true, write true. If the statement is false, change the underlined word or words to make the statement true. 1. _____________ An atom’s valence electrons are those electrons that have the highest energy. 2. _____________ Atoms tend to be stable and nonreactive if they have six va ...
studyguideperiodictrends
... _____ and chemical properties___. Bonus: What scientist is given the most credit because he published his info first? Mendeleev ...
... _____ and chemical properties___. Bonus: What scientist is given the most credit because he published his info first? Mendeleev ...
Periodic Table How did Dmitri Mendeleev arrange the periodic table?
... • As you go from left to right, atomic number increases by 1 – number of protons increases by 1 – number of electrons also increases by 1 in the same valence shell ...
... • As you go from left to right, atomic number increases by 1 – number of protons increases by 1 – number of electrons also increases by 1 in the same valence shell ...
Review Packet - Old Saybrook Public Schools
... 10. Those electrons that are largely responsible for an atom's chemical behavior are called b. high energy electrons a. core electrons c. stable electrons b. valence electrons 1L. The second period of the periodic table contains c.s, p, and d-block elements a. s-block elements d. p block elements b. ...
... 10. Those electrons that are largely responsible for an atom's chemical behavior are called b. high energy electrons a. core electrons c. stable electrons b. valence electrons 1L. The second period of the periodic table contains c.s, p, and d-block elements a. s-block elements d. p block elements b. ...
PPT format
... B, Al, Ga, In, Tl C, Si, Ge, Sn, Pb N, P, As, Sb, Bi O, S, Se, Te, Po F, Cl, Br, I, At (He), Ne, Ar, Kr, Xe, Rn ...
... B, Al, Ga, In, Tl C, Si, Ge, Sn, Pb N, P, As, Sb, Bi O, S, Se, Te, Po F, Cl, Br, I, At (He), Ne, Ar, Kr, Xe, Rn ...
The Periodic Table notes
... valence electrons (electrons that are in the outermost shell of the atom) ...
... valence electrons (electrons that are in the outermost shell of the atom) ...
View PDF
... 16. Mendeleev and Alien Periodic Tables: Recognize how to identify unknown elements based on common physical and chemical properties across the periods and down the groups. ...
... 16. Mendeleev and Alien Periodic Tables: Recognize how to identify unknown elements based on common physical and chemical properties across the periods and down the groups. ...
The Periodic Table
... an airfoil through the air in order to produce lift, aerostatic craft such as airships (and balloons) stay aloft primarily by means of a cavity (usually quite large) filled with a gas of lesser density than the surrounding atmosphere. ...
... an airfoil through the air in order to produce lift, aerostatic craft such as airships (and balloons) stay aloft primarily by means of a cavity (usually quite large) filled with a gas of lesser density than the surrounding atmosphere. ...
Brown, Le May, and Bursten: Chapter 2
... Law of definite proportions led to theory that all matter made up of atoms. 1. Atoms- basic building blocks and don't change when react with other atoms. 2. Element- describes matter composed of only one type of atom. 3. Compound- combination of atoms in specific proportions. 4. Chemical reaction- a ...
... Law of definite proportions led to theory that all matter made up of atoms. 1. Atoms- basic building blocks and don't change when react with other atoms. 2. Element- describes matter composed of only one type of atom. 3. Compound- combination of atoms in specific proportions. 4. Chemical reaction- a ...
Reinforcing Key Concepts
... Sometimes, an atom’s nucleus may have too many or too few neutrons to be stable. When this occurs, the atom will produce particles and energy until it is again stable. If the number of protons in the nucleus changes, the identity of the atom changes. Atoms that change identity are said to be radioac ...
... Sometimes, an atom’s nucleus may have too many or too few neutrons to be stable. When this occurs, the atom will produce particles and energy until it is again stable. If the number of protons in the nucleus changes, the identity of the atom changes. Atoms that change identity are said to be radioac ...
u4ohnotes18f2005 - Teach-n-Learn-Chem
... Periodicity there are trends in properties of elements -- left-right AND up-down trends atomic radius: the size of a neutral atom …increases as we go add a new energy level each time …decreases as we go coulombic attraction: attraction between (+) and (–) Depends on… amount of charge ...
... Periodicity there are trends in properties of elements -- left-right AND up-down trends atomic radius: the size of a neutral atom …increases as we go add a new energy level each time …decreases as we go coulombic attraction: attraction between (+) and (–) Depends on… amount of charge ...
Unit 4 Notes - Teach-n-Learn-Chem
... Periodicity there are trends in properties of elements -- left-right AND up-down trends atomic radius: the size of a neutral atom …increases as we go add a new energy level each time …decreases as we go coulombic attraction: attraction between (+) and (–) Depends on… amount of charge ...
... Periodicity there are trends in properties of elements -- left-right AND up-down trends atomic radius: the size of a neutral atom …increases as we go add a new energy level each time …decreases as we go coulombic attraction: attraction between (+) and (–) Depends on… amount of charge ...
Chemistry: Unit 4 - Teach-n-Learn-Chem
... Periodicity there are trends in properties of elements -- left-right AND up-down trends atomic radius: the size of a neutral atom …increases as we go add a new energy level each time …decreases as we go coulombic attraction: attraction between (+) and (–) Depends on… amount of charge ...
... Periodicity there are trends in properties of elements -- left-right AND up-down trends atomic radius: the size of a neutral atom …increases as we go add a new energy level each time …decreases as we go coulombic attraction: attraction between (+) and (–) Depends on… amount of charge ...
Lecture 3 - TCD Chemistry
... Is the energy that is necessary to remove an electron from an atom or a molecule. The Ionisation Energy is a measure for the force by which an electron is bound in the atom. The Ionisation Energy is a function of the radius and the charge of an atom: ...
... Is the energy that is necessary to remove an electron from an atom or a molecule. The Ionisation Energy is a measure for the force by which an electron is bound in the atom. The Ionisation Energy is a function of the radius and the charge of an atom: ...
E/F Physical Science Learning Targets ALL Name: Date: Hour
... 2. Elements within a group have the ___________________ number of valence electrons. 3. The reactivity of alkali metals ____________________ from the top of Group 1A to the bottom. 4. Sodium is stored under oil because it _________________________________. 5. Differences in reactivity among alkaline ...
... 2. Elements within a group have the ___________________ number of valence electrons. 3. The reactivity of alkali metals ____________________ from the top of Group 1A to the bottom. 4. Sodium is stored under oil because it _________________________________. 5. Differences in reactivity among alkaline ...
Periodic Table
... shell but with increasing nuclear charge. The increasing number of protons (higher Z) attracts the electrons more, making it harder to remove an electron from the atom-hence a higher IE. Vertical: As you go down a group from top to bottom, you always have the same valence shell configuration. Howeve ...
... shell but with increasing nuclear charge. The increasing number of protons (higher Z) attracts the electrons more, making it harder to remove an electron from the atom-hence a higher IE. Vertical: As you go down a group from top to bottom, you always have the same valence shell configuration. Howeve ...
File - McArthur Media
... • Elements that are reactive bond easily with other elements to make compounds. • Some elements are only found in nature bonded with other elements. • What makes an element reactive? – An incomplete valence electron level. – All atoms (except hydrogen and helium) want to have 8 electrons in their ve ...
... • Elements that are reactive bond easily with other elements to make compounds. • Some elements are only found in nature bonded with other elements. • What makes an element reactive? – An incomplete valence electron level. – All atoms (except hydrogen and helium) want to have 8 electrons in their ve ...
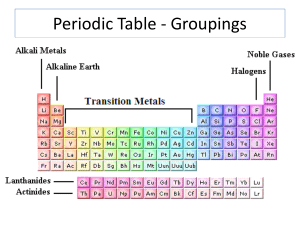
Reading the Periodic Table - Science
... A way of organizing & classifying elements according to their PROPERTIES • Arranged in rows and columns ...
... A way of organizing & classifying elements according to their PROPERTIES • Arranged in rows and columns ...
The Periodic Table - Science Education at Jefferson Lab
... • Elements that are reactive bond easily with other elements to make compounds. • Some elements are only found in nature bonded with other elements. • What makes an element reactive? – An incomplete valence electron level. – All atoms (except hydrogen and helium) want to have 8 electrons in their ve ...
... • Elements that are reactive bond easily with other elements to make compounds. • Some elements are only found in nature bonded with other elements. • What makes an element reactive? – An incomplete valence electron level. – All atoms (except hydrogen and helium) want to have 8 electrons in their ve ...
The Periodic Table of Elements - PAMS-Doyle
... • Family members include: chlorine, fluorine, bromine, iodine, and astatine ...
... • Family members include: chlorine, fluorine, bromine, iodine, and astatine ...
Noble gas

The noble gases make a group of chemical elements with similar properties. Under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six noble gases that occur naturally are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).For the first six periods of the periodic table, the noble gases are exactly the members of group 18 of the periodic table.It is possible that due to relativistic effects, the group 14 element flerovium exhibits some noble-gas-like properties, instead of the group 18 element ununoctium. Noble gases are typically highly unreactive except when under particular extreme conditions. The inertness of noble gases makes them very suitable in applications where reactions are not wanted. For example: argon is used in lightbulbs to prevent the hot tungsten filament from oxidizing; also, helium is breathed by deep-sea divers to prevent oxygen and nitrogen toxicity.The properties of the noble gases can be well explained by modern theories of atomic structure: their outer shell of valence electrons is considered to be ""full"", giving them little tendency to participate in chemical reactions, and it has been possible to prepare only a few hundred noble gas compounds. The melting and boiling points for a given noble gas are close together, differing by less than 10 °C (18 °F); that is, they are liquids over only a small temperature range.Neon, argon, krypton, and xenon are obtained from air in an air separation unit using the methods of liquefaction of gases and fractional distillation. Helium is sourced from natural gas fields which have high concentrations of helium in the natural gas, using cryogenic gas separation techniques, and radon is usually isolated from the radioactive decay of dissolved radium, thorium, or uranium compounds (since those compounds give off alpha particles). Noble gases have several important applications in industries such as lighting, welding, and space exploration. A helium-oxygen breathing gas is often used by deep-sea divers at depths of seawater over 55 m (180 ft) to keep the diver from experiencing oxygen toxemia, the lethal effect of high-pressure oxygen, and nitrogen narcosis, the distracting narcotic effect of the nitrogen in air beyond this partial-pressure threshold. After the risks caused by the flammability of hydrogen became apparent, it was replaced with helium in blimps and balloons.