4-6 Making Artificial Fragrances Lab fy11
... alcohol, and RCOOR’ represents an ester. Esters are named by using the alcohol name with the acid name after its suffix has been changed to ‘-ate’. For example, ethyl alcohol and acetic acid produce the ester ethyl acetate. In this lab, you will prepare three types of fragrant ester molecules from t ...
... alcohol, and RCOOR’ represents an ester. Esters are named by using the alcohol name with the acid name after its suffix has been changed to ‘-ate’. For example, ethyl alcohol and acetic acid produce the ester ethyl acetate. In this lab, you will prepare three types of fragrant ester molecules from t ...
8. Acids and bases
... dissociated into their ions in aqueous solution: HCl (aq) → H+(aq) + Cl- (aq) • Monoprotic acids: 1 mol of acid gives 1 mol of hydrogen ions • Diprotic acids: 1 mol of acid gives 2 mol of hydrogen ions ...
... dissociated into their ions in aqueous solution: HCl (aq) → H+(aq) + Cl- (aq) • Monoprotic acids: 1 mol of acid gives 1 mol of hydrogen ions • Diprotic acids: 1 mol of acid gives 2 mol of hydrogen ions ...
INTRODUCTION
... 2. Prepare a warm water bath use the thermometers (max 80 degrees C) 3. Refer to the observation table attached. To test tubes add 1.0mL (about 0.75cm) of the appropriate acid 4. Refer to the observation table attached. To each test tube add 10 drops of the appropriate alcohol 5. To each test tube a ...
... 2. Prepare a warm water bath use the thermometers (max 80 degrees C) 3. Refer to the observation table attached. To test tubes add 1.0mL (about 0.75cm) of the appropriate acid 4. Refer to the observation table attached. To each test tube add 10 drops of the appropriate alcohol 5. To each test tube a ...
Esters - chymist.com
... In contrast to inorganic reactions, which usually involve metals combining with non-metals or polyatomic ions, organic reactions occur as additions or substitutions into a compound or as a condensation-type reaction where removal of certain atoms or groups cause two molecules to combine. One type of ...
... In contrast to inorganic reactions, which usually involve metals combining with non-metals or polyatomic ions, organic reactions occur as additions or substitutions into a compound or as a condensation-type reaction where removal of certain atoms or groups cause two molecules to combine. One type of ...
Ester Lab / Adobe Acrobat Document
... 2. Prepare a warm water bath use the thermometers (max 80 degrees C) 3. Refer to the observation table attached. To test tubes add 1.0mL (about 0.75cm) of the appropriate acid 4. Refer to the observation table attached. To each test tube add 10 drops of the appropriate alcohol 5. To each test tube a ...
... 2. Prepare a warm water bath use the thermometers (max 80 degrees C) 3. Refer to the observation table attached. To test tubes add 1.0mL (about 0.75cm) of the appropriate acid 4. Refer to the observation table attached. To each test tube add 10 drops of the appropriate alcohol 5. To each test tube a ...
Acids and Bases - Parkway C-2
... Honors Chemistry For the following reactions, classify them as: o acid ionization o base ionization o proton transfer o neutralization (you may have more than one answer for a reaction) ...
... Honors Chemistry For the following reactions, classify them as: o acid ionization o base ionization o proton transfer o neutralization (you may have more than one answer for a reaction) ...
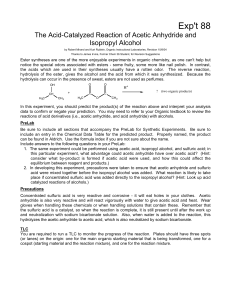
The Acid-Catalyzed Reaction of Acetic
... Ester syntheses are one of the more enjoyable experiments in organic chemistry, as one can't help but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, ...
... Ester syntheses are one of the more enjoyable experiments in organic chemistry, as one can't help but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, ...
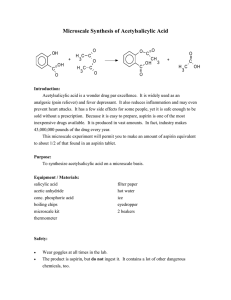
Microscale Synthesis of Acetylsalicylic Acid
... hot (but not boiling) water. The temperature must be between 70 and 90C. 4. After several minutes of heating, cautiously add 0.2 mL of water to decompose the excess acetic anhydride. When you observe no more evidence of reaction, add 0.3 mL more water; remove the tube from the hot water bath and al ...
... hot (but not boiling) water. The temperature must be between 70 and 90C. 4. After several minutes of heating, cautiously add 0.2 mL of water to decompose the excess acetic anhydride. When you observe no more evidence of reaction, add 0.3 mL more water; remove the tube from the hot water bath and al ...
Chapter 14 Applying Neutralizaton Titrations
... primary-standard purity, so standardization is required after preparation. The Effect of Carbon Dioxide In solution as well as in the solid state, the hydroxides of sodium, potassium, and barium react rapidly with atmospheric carbon dioxide to produce the corresponding carbonate: CO2 + 2OHCO32- + H2 ...
... primary-standard purity, so standardization is required after preparation. The Effect of Carbon Dioxide In solution as well as in the solid state, the hydroxides of sodium, potassium, and barium react rapidly with atmospheric carbon dioxide to produce the corresponding carbonate: CO2 + 2OHCO32- + H2 ...
synthesizing esters in the laboratory
... cucumber, and raspberry. Esters are easily made in the laboratory from their corresponding carboxylic acid and a condensation reaction with an alcohol. This reaction is condensed and catalyzed with concentrated (18M) sulfuric acid. The making of esters in the school laboratory is also an excellent w ...
... cucumber, and raspberry. Esters are easily made in the laboratory from their corresponding carboxylic acid and a condensation reaction with an alcohol. This reaction is condensed and catalyzed with concentrated (18M) sulfuric acid. The making of esters in the school laboratory is also an excellent w ...
Slide 1
... Given samples of various alcohols and carboxylic acids Establish some of their physical and chemical properties & perform various reactions HO HO ethanol ...
... Given samples of various alcohols and carboxylic acids Establish some of their physical and chemical properties & perform various reactions HO HO ethanol ...
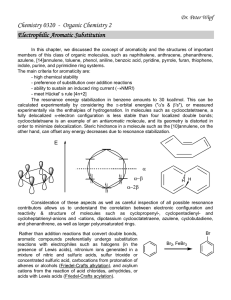
In this chapter, alkanes, alkenes, alkynes
... contributors allows us to understand the correlation between electronic configuration and reactivity & structure of molecules such as cyclopropenyl-, cyclopentadienyl- and cycloheptatrienyl-anions and -cations, dipotassium cyclooctatetraene, azulene, cyclobutadiene, and phenanthrene, as well as larg ...
... contributors allows us to understand the correlation between electronic configuration and reactivity & structure of molecules such as cyclopropenyl-, cyclopentadienyl- and cycloheptatrienyl-anions and -cations, dipotassium cyclooctatetraene, azulene, cyclobutadiene, and phenanthrene, as well as larg ...
acids: bases - IDS-chem2-Rn-10
... that the pH (acidity or basicity) of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. ...
... that the pH (acidity or basicity) of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. ...
Titration Worksheet
... Titration Worksheet CK12 Chemistry Name _____________________________ Date ____________ Show ALL your work in solving these problems. ...
... Titration Worksheet CK12 Chemistry Name _____________________________ Date ____________ Show ALL your work in solving these problems. ...
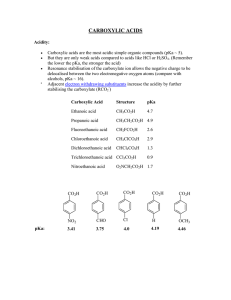
carboxylic acids - La Salle University
... 1o and 2o alkyl halides (X = Cl, Br, I) or tosylates undergo SN2 substitution with cyanide salts to give nitriles. Nitriles can be hydrolysed to carboxylic acids without the isolation of the amide intermediate. Note that the carbon skeleton is extended by 1 C atom during this reaction sequence. Alth ...
... 1o and 2o alkyl halides (X = Cl, Br, I) or tosylates undergo SN2 substitution with cyanide salts to give nitriles. Nitriles can be hydrolysed to carboxylic acids without the isolation of the amide intermediate. Note that the carbon skeleton is extended by 1 C atom during this reaction sequence. Alth ...
Document
... Asyou perform the experiment, record your obsmwations ii~ Table 50.1. 1. CAUTION: The acids used in this experime~tt are extremdy corrosive. Label five medium test tubes with the nnmbers 1-5. Put the robes in a test-tube rack. To each of the tubes, add 1 mL of a carboxylic acid and 1 mL of an alcoho ...
... Asyou perform the experiment, record your obsmwations ii~ Table 50.1. 1. CAUTION: The acids used in this experime~tt are extremdy corrosive. Label five medium test tubes with the nnmbers 1-5. Put the robes in a test-tube rack. To each of the tubes, add 1 mL of a carboxylic acid and 1 mL of an alcoho ...
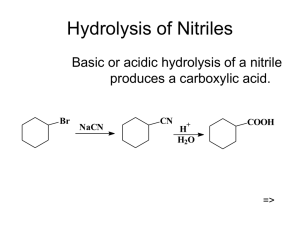
Hydrolysis of Nitriles
... Amine (base) removes a proton from the carboxylic acid to form a salt. Heating the salt above 100C drives off steam and forms the amide. O ...
... Amine (base) removes a proton from the carboxylic acid to form a salt. Heating the salt above 100C drives off steam and forms the amide. O ...
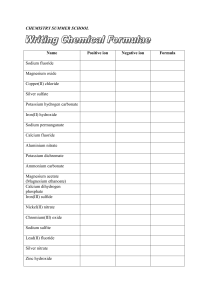
chemistry summer school
... Silver nitrate and copper(II) chloride solutions combine to form solid silver chloride and a solution of copper(II) nitrate. Sulfuric acid reacts with aluminium oxide powder. Glucose (C6H12O6) is fermented to carbon dioxide and ethanol (C2H5OH) Lead(II) nitrate and potassium iodide solutions combine ...
... Silver nitrate and copper(II) chloride solutions combine to form solid silver chloride and a solution of copper(II) nitrate. Sulfuric acid reacts with aluminium oxide powder. Glucose (C6H12O6) is fermented to carbon dioxide and ethanol (C2H5OH) Lead(II) nitrate and potassium iodide solutions combine ...
7.2: Properties, Names, and Formulas page 268 •Acids and bases
... 7.2: Properties, Names, and Formulas ...
... 7.2: Properties, Names, and Formulas ...
Nomenclature of Acids and Complex ions
... a molecule is soluble in water – Hydrocarbons are only slightly polar and tend to be insoluble in water These ...
... a molecule is soluble in water – Hydrocarbons are only slightly polar and tend to be insoluble in water These ...
OVERVIEW DESCRIPTION DES ADVANTAGES LICENSING
... Lactic acid has been an intermediate-volume specialty chemical for a wide range of food processing and industrial applications that includes pharmaceutical, cosmetics and other chemicals synthesis. Researchers at the South Dakota School of Mines & Technology have developed a unique approach to synth ...
... Lactic acid has been an intermediate-volume specialty chemical for a wide range of food processing and industrial applications that includes pharmaceutical, cosmetics and other chemicals synthesis. Researchers at the South Dakota School of Mines & Technology have developed a unique approach to synth ...
Sulfuric acid
Sulfuric acid (alternative spelling sulphuric acid) is a highly corrosive strong mineral acid with the molecular formula H2SO4 and molecular weight 98.079 g/mol. It is a pungent-ethereal, colorless to slightly yellow viscous liquid which is soluble in water at all concentrations. Sometimes, it is dyed dark brown during production to alert people to its hazards. The historical name of this acid is oil of vitriol.Sulfuric acid is a diprotic acid and shows different properties depending upon its concentration. Its corrosiveness on other materials, like metals, living tissues or even stones, can be mainly ascribed to its strong acidic nature and, if concentrated, strong dehydrating and oxidizing properties. Sulfuric acid at a high concentration can cause very serious damage upon contact, since not only does it cause chemical burns via hydrolysis, but also secondary thermal burns through dehydration. It can lead to permanent blindness if splashed onto eyes and irreversible damage if swallowed. Accordingly, safety precautions should be strictly observed when handling it. Moreover, it is hygroscopic, readily absorbing water vapour from the air.Sulfuric acid has a wide range of applications including domestic acidic drain cleaner, electrolyte in lead-acid batteries and various cleaning agents. It is also a central substance in the chemical industry. Principal uses include mineral processing, fertilizer manufacturing, oil refining, wastewater processing, and chemical synthesis. It is widely produced with different methods, such as contact process, wet sulfuric acid process and some other methods.