* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download INTRODUCTION
Survey
Document related concepts
Transcript
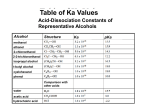
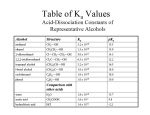
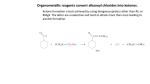
ORGANIC SYNTHESIS LAB Chemistry - KCVI INTRODUCTION In this lab you will remind yourself about how we produce carboxylic acids and you will carry out examples of the synthesis of an ester. Oxidation- In organic chemistry, oxidation usually involves the removal of hydrogen atoms from the hydrocarbon (often accompanied by the addition of a highly electronegative element such as oxygen, chlorine or nitrogen to the compound) Reduction of organic compounds virtually always involves the addition of hydrogen and frequently the removal of electronegative elements such as oxygen. PART I – THOUGHT LAB - OXIDATION OF ALCOHOLS Primary alcohols have the OH attached at the end of the hydrocarbon chain. A primary alcohol may be oxidized in stages to an aldehyde and then to a carboxylic acid. A strong oxidizing agent such as dichromic acid is required. In stage 1 the primary alcohol is oxidized to an aldehyde and in stage 2 the aldehyde is further oxidized to a carboxylic (alkan-oic) acid. The general reaction may be represented as: Stage 1: catalyst primary alcohol oxidizing agent aldehyde waterr primary alcohol Stage 2: + dichromate aldehyde + Cr(III) ion catalyst aldehyde oxidizing agent carboxylic acid water O O H2SO4 + R C (O) R C H2O + H OH You may be interested to know that this reaction is the basis for the “breathalyzer test". The degree of colour change of the amber dichromate ion to the green chromium(III) can be quantitatively interpreted to determine blood alcohol (ethanol) levels. Second generation breathalyzer tests measure the amount of water produced in the reaction, for a more accurate measure. Secondary alcohols (OH attached to a non-terminal carbon) may be oxidized in a single stage to the corresponding ketone. The general reaction is: secondary alcohol + oxidizing agent ketone R' R C OH H + (O) H2SO4 R' R C O + H2O Tertiary alcohols have the OH attached to a carbon that is in turn attached to 3 other carbons. These are generally quite stable since the OH group is surrounded by three alkyl groups which protect it from chemical attack. Tertiary alcohols are not readily oxidized as there are no hydrogen atoms on the carbon containing the -OH group. Imaginary THOUGHT Procedure (don’t actually perform this): 1. Pour 5 mL of sodium dichromate/sulfuric acid solution into each of three 18x150 mm test tubes (CAUTION!: CORROSIVE LIQUID) FACE SHIELDS AND APRONS!! ) 2. To the first test tube add 5-6 drops of ethanol (a primary alcohol). Using a test tube holder, warm gently in the "cool" part of a Bunsen burner flame by waving the tube slowly back and forth. Warm until a definite colour change is noted. CAUTION: CONTENTS COULD BOIL OVER IF HEATED TOO STRONGLY! ! POINT TEST TUBE AT 45° TOWARD A BLANK WALL, AWAY FROM OTHER STUDENTS! DO NOT BOIL! Note the odour (remove face shield and waft odour gently towards your nose) and colour change. Do you recognize the odour? 3. Add 5-6 drops of 2-propanol (a secondary alcohol) to the second test tube and warm gently in the same manner. Note colour change and odour. 4. Add 5-6 drops of 2-methyl-2-propanol (a tertiary alcohol) to the third test tube and repeat the procedure. Note any changes. ANALYSIS: Write balanced structural equations for the oxidation reactions that would occur in the lab. Name the products using IUPAC nomenclature. 1 PART II – REAL - SYNTHESIS OF ESTERS Many esters are found naturally in fruits, and are responsible for some of their pleasant odours. Synthetic esters are produced from condensation reactions between alcohols and carboxylic acids. In this activity you will synthesize, and detect the odours of, several esters. Glacial acetic acid , Concentrated sulfuric acid, Benzoic Acid and Salicylic Acids are corrosive. Avoid contact with skin and eyes. It is also volatile, so you must be careful to avoid inhalation. Wear eye protection and a laboratory apron. All alcohols are highly flammable. Do not use near an open flame. Alcohols react with carboxylic acids to produce esters. Water is produced as well. This is the organic equivalent of a neutralization reaction. The reaction is catalysed by an acid. Esters are generally noted for their pleasant odours. General Equation: carboxylic acid (alkanoic acid) + alcohol alkanoic acid alkanol acid ester O R C O + HO R' + water alkyl alkanoate R C OH + H2O (H from the alcohol + OH from the acid) O R' PROCEDURE 1. Number eight clean test tubes. DO ONLY 3-4 tests at once and LABEL Test Tubes as you go!!! 2. Prepare a warm water bath use the thermometers (max 80 degrees C) 3. Refer to the observation table attached. To test tubes add 1.0mL (about 0.75cm) of the appropriate acid 4. Refer to the observation table attached. To each test tube add 10 drops of the appropriate alcohol 5. To each test tube add 2 drops of Concentrated Sulfuric Acid 6. Using test tube tongs, put the test tubes in the warm water bath and heat for 5 minutes… DO NOT LET THE MIXTURES BOIL. Remove the test tubes and adjust the temperature down. 7. After 5 minutes, remove each test tube, WAFT to note the smell and record in your observations chart. 8. Add 0.5mL (0.5cm) of COLD water and mix. WAFT and note the smell immediately 9. Discard the solution down the drain with water. 10. REPEAT using the chemicals listed to complete the observation table Analysis and Conclusion: 1. Write balanced structural equations for the reactions observed. What are the structural formulae and IUPAC names for each product produced. 2. Explain why Sulfuric acid is or is not included in the chemical equations 3. What sources of Random error and systematic error did you observe in this experiment. Suggest improvements for the lab. 4. Explain step by step the Reaction Mechanism of an esterification reaction – “How does the reaction actually happen?” draw chemical structures to help show the mechanism. 5. Research and identify three uses or applications of esters – be sure to include at least ONE ester from those you have created. Benzioc Acid C6H5COOH Acid Salicylic 2 Alcohol Carboxylic Acid Methanol Salicylic Acid Methanol Benzoic Acid Ethanol Salicylic Acid Ethanol Benzoic Acid 2-Methyl propanol Ethanioc Acid 1- Butanol Salicylic Acid 1- Butanol Ethanioc Acid 1- Pentanol Ethanioc Acid Smell during heating Smell after water added Ester produced Structure of ester 3 4