Exercise 2 PARTITION COEFFICIENT OF SUCCINIC ACID
... The dissolved substance irrespective of its total amount, distributes itself between the two layers in a constant concentration ratio, at constant temperature. The ratio, equal to the constant in Equation 1 is reffered as the partition constant or partition coefficient (k) (or sometime designated as ...
... The dissolved substance irrespective of its total amount, distributes itself between the two layers in a constant concentration ratio, at constant temperature. The ratio, equal to the constant in Equation 1 is reffered as the partition constant or partition coefficient (k) (or sometime designated as ...
Laser Refraction and Diffraction
... numerous light point sources. Constructive interference caused by numerous light point sources occurs only at specific angles: d sin θ = mλ , m = integers ...
... numerous light point sources. Constructive interference caused by numerous light point sources occurs only at specific angles: d sin θ = mλ , m = integers ...
Are You suprised ?
... A system at equilibrium is described by the equation: Heat + SO2Cl2(l ) ⇌ SO2(g) + Cl2(g) One of the following sentences is correct. A) Adding Cl2 will increase heat. B) The equilibrium will move to the left when we remove Cl2. C) Increasing the pressure has no effect on this system. D) Adding catal ...
... A system at equilibrium is described by the equation: Heat + SO2Cl2(l ) ⇌ SO2(g) + Cl2(g) One of the following sentences is correct. A) Adding Cl2 will increase heat. B) The equilibrium will move to the left when we remove Cl2. C) Increasing the pressure has no effect on this system. D) Adding catal ...
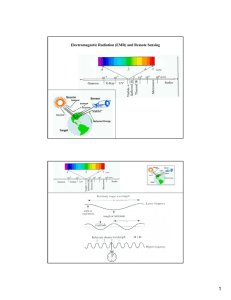
Electromagnetic Radiation (EMR) and Remote Sensing
... Happened when the lower atmosphere contains suspended aerosols. The aerosols should have diameters at least 10 times larger than the wavelengths under consideration. The important scattering agents include large particles of smoke, water vapor, water droplets, ice crystals in the clouds and fog. Non ...
... Happened when the lower atmosphere contains suspended aerosols. The aerosols should have diameters at least 10 times larger than the wavelengths under consideration. The important scattering agents include large particles of smoke, water vapor, water droplets, ice crystals in the clouds and fog. Non ...
light - Churchill High School
... The light has to travels through more atmosphere during sunrise and sunset because the distance that the light has to travel from the Sun to an observer is at its greatest. Blue/violet light is scattered the most & red light is scattered the least. This means the large amount of blue and violet ligh ...
... The light has to travels through more atmosphere during sunrise and sunset because the distance that the light has to travel from the Sun to an observer is at its greatest. Blue/violet light is scattered the most & red light is scattered the least. This means the large amount of blue and violet ligh ...
General Equilibrium
... where K is the equilibrium constant, and aX is the activity of X and described by the activity coefficient X and [X]: aX = X[X] In dilute solutions, the activity coefficient approaches unity. Often, experimental conditions allow us to assume activity coefficients of one so that concentrations can ...
... where K is the equilibrium constant, and aX is the activity of X and described by the activity coefficient X and [X]: aX = X[X] In dilute solutions, the activity coefficient approaches unity. Often, experimental conditions allow us to assume activity coefficients of one so that concentrations can ...
Eyewear Lens Selection Guide
... infrared radiation generated when working with molten metal, and in welding, cutting, soldering and brazing. ...
... infrared radiation generated when working with molten metal, and in welding, cutting, soldering and brazing. ...
Reduced absorption coefficient (RAC)
... The optical depth of a uniform layer of thickness L, alkali number density NA , helium number density NHe: ...
... The optical depth of a uniform layer of thickness L, alkali number density NA , helium number density NHe: ...
of the Physical and Technical Faculty
... The course "Laboratory Practicum on Optical Devices" is destined to the study of the optical techniques, devices and its applications, most important effects and laws. The course consists of laboratory works and seminars. The seminars are primarily intended to familiarize with the studied optical ph ...
... The course "Laboratory Practicum on Optical Devices" is destined to the study of the optical techniques, devices and its applications, most important effects and laws. The course consists of laboratory works and seminars. The seminars are primarily intended to familiarize with the studied optical ph ...
Photonic Devices I Purpose of the Lab
... 5) Use the EXFO optical power meter to measure the output power of the laser. Make sure to set the power meter to the peak wavelength: - To set the readings unit press: dBm/Watt. - Press the λ key to view the available wavelengths. - To add a new wavelength: SETUP → arrow “→” button → LAMBDA → arrow ...
... 5) Use the EXFO optical power meter to measure the output power of the laser. Make sure to set the power meter to the peak wavelength: - To set the readings unit press: dBm/Watt. - Press the λ key to view the available wavelengths. - To add a new wavelength: SETUP → arrow “→” button → LAMBDA → arrow ...
LITHIUM, SODIUM, AND POTASSIUM RESONANCE LINES
... The optical depth of a uniform layer of thickness L, alkali number density NA , helium number density NHe: ...
... The optical depth of a uniform layer of thickness L, alkali number density NA , helium number density NHe: ...
Chapter 23 notes
... dichroism: the selective absorption of one of the polarized components much more strongly than the other polarizing axis: the axis in which the light is polarized parallel to Light transmitted by polarizing filter When linearly polarized light strikes a polarizing filter with its axis at an angle φ ...
... dichroism: the selective absorption of one of the polarized components much more strongly than the other polarizing axis: the axis in which the light is polarized parallel to Light transmitted by polarizing filter When linearly polarized light strikes a polarizing filter with its axis at an angle φ ...
1 Introduction to Electromagnetic Waves 2 Speed of an
... dichroism: the selective absorption of one of the polarized components much more strongly than the other polarizing axis: the axis in which the light is polarized parallel to Light transmitted by polarizing filter When linearly polarized light strikes a polarizing filter with its axis at an angle φ ...
... dichroism: the selective absorption of one of the polarized components much more strongly than the other polarizing axis: the axis in which the light is polarized parallel to Light transmitted by polarizing filter When linearly polarized light strikes a polarizing filter with its axis at an angle φ ...
homework assignment 2 - the Petersen Home Page
... 1. A 15.40-g sample of a finely-divided mixture of only Fe2S3 and FeS was reacted with excess H2 at elevated temperatures. If the weight percent of Fe2S3 in this mixture is 57.4%, then calculate the total mass in grams of Fe that can be produced. Assume the only other product of these reactions is H ...
... 1. A 15.40-g sample of a finely-divided mixture of only Fe2S3 and FeS was reacted with excess H2 at elevated temperatures. If the weight percent of Fe2S3 in this mixture is 57.4%, then calculate the total mass in grams of Fe that can be produced. Assume the only other product of these reactions is H ...
"Is affirmed"
... Electronic transitions involve shifts of electrons from one quantum shell to another. A photon of a specific wavelength may induce an electron to move from a lower orbit to a higher one, thereby allowing the EMR energy to be absorbed. The wavelengths that can cause electronic transitions are determi ...
... Electronic transitions involve shifts of electrons from one quantum shell to another. A photon of a specific wavelength may induce an electron to move from a lower orbit to a higher one, thereby allowing the EMR energy to be absorbed. The wavelengths that can cause electronic transitions are determi ...
Ultraviolet–visible spectroscopy

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared [NIR]) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.