Thermodynamics Guided Notes
... missed in class. It is very important to keep up with the reading and homework from this unit since we don’t have much time to cover it and because you’ll learn so much more that way! ...
... missed in class. It is very important to keep up with the reading and homework from this unit since we don’t have much time to cover it and because you’ll learn so much more that way! ...
Specific Heat of a Metal
... Report for Experiment 41 Specific Heat of a Metal Prelaboratory Questions 1. Since the specific heat of water is given in units of joules per gram degree Celsius why do we measure the volume of water in the calorimeter instead of its mass? 2. A 22.50 g piece of an unknown metal is heated to 100oC th ...
... Report for Experiment 41 Specific Heat of a Metal Prelaboratory Questions 1. Since the specific heat of water is given in units of joules per gram degree Celsius why do we measure the volume of water in the calorimeter instead of its mass? 2. A 22.50 g piece of an unknown metal is heated to 100oC th ...
Electrical Equivalent of Heat
... The last step is to divide both sides of the new equation by the heat energy (numerical value only) this should yield something similar to the accepted electrical equivalent of heat seen below: 1 calorie = 4.186 Joules ...
... The last step is to divide both sides of the new equation by the heat energy (numerical value only) this should yield something similar to the accepted electrical equivalent of heat seen below: 1 calorie = 4.186 Joules ...
Heat Transfer Oil
... In many industrial applications heating is provided indirectly by circulating hot oil through a heat exchanger, thus reducing hot spots and increasing the safety of the heating process. In quenching applications, heat is required to be rapidly drawn away from the parts in contact with the oil. Due t ...
... In many industrial applications heating is provided indirectly by circulating hot oil through a heat exchanger, thus reducing hot spots and increasing the safety of the heating process. In quenching applications, heat is required to be rapidly drawn away from the parts in contact with the oil. Due t ...
Schaums Heat
... 5. A thermos bottle contains 250 g of coffee at 900C. To this is added 20g of milk at 50C. After equilibrium is established, what is the temperature of the liquid? 6. A thermos bottle contains 150 g of water at 40C. Into this is placed 90g of metal at 1000C. After equilibrium is established, the tem ...
... 5. A thermos bottle contains 250 g of coffee at 900C. To this is added 20g of milk at 50C. After equilibrium is established, what is the temperature of the liquid? 6. A thermos bottle contains 150 g of water at 40C. Into this is placed 90g of metal at 1000C. After equilibrium is established, the tem ...
electrical heating - Home - KSU Faculty Member websites
... A heat pump uses an electric motor to drive a refrigeration cycle, drawing heat from a source such as ground water or outside air and directing it into the space to be warmed. Such systems can deliver two or three units of heating energy for every unit of purchased energy. ...
... A heat pump uses an electric motor to drive a refrigeration cycle, drawing heat from a source such as ground water or outside air and directing it into the space to be warmed. Such systems can deliver two or three units of heating energy for every unit of purchased energy. ...
Chapters 1 and 2
... Temperature is the thing that’s the same for two objects, after they’ve been in contact long enough. Long enough so that the two objects are in thermal equilibrium. Time required to reach thermal equilibrium is the relaxation time. Temperature is usually measured in K, C or F and cannot be expres ...
... Temperature is the thing that’s the same for two objects, after they’ve been in contact long enough. Long enough so that the two objects are in thermal equilibrium. Time required to reach thermal equilibrium is the relaxation time. Temperature is usually measured in K, C or F and cannot be expres ...
Current_Energy_Sustainable_Solutions
... Perspective: If I ride my bike 17 mph I can generate 100 Watts of power. If Everybody rode their bike for an 100 Watts then the world power would be 6.5 x 1011 W or .65 TW ...
... Perspective: If I ride my bike 17 mph I can generate 100 Watts of power. If Everybody rode their bike for an 100 Watts then the world power would be 6.5 x 1011 W or .65 TW ...
Heat Calculations with Specific Heat
... Specific Heat varies depending on: Type of substance State of matter of substance (s, l, g) Temperature of the reaction ...
... Specific Heat varies depending on: Type of substance State of matter of substance (s, l, g) Temperature of the reaction ...
Thermal Energy
... Heat Transfer Heat is the Transfer of Thermal Energy from an object Of higher temperature to an object of lower temperature • Heat does NOT transfer randomly • Heat only travels in ONE direction ...
... Heat Transfer Heat is the Transfer of Thermal Energy from an object Of higher temperature to an object of lower temperature • Heat does NOT transfer randomly • Heat only travels in ONE direction ...
14_Water Cooling System
... 6. COOLING WATER TREATMENT & CONSEQUENCES If the cooling water is not properly treated, the closed cooling systems may undergo fouling, formation of deposits ( preventing or disturbing the heat transfer ). The deposit consists of loose sludge and solid particles. ...
... 6. COOLING WATER TREATMENT & CONSEQUENCES If the cooling water is not properly treated, the closed cooling systems may undergo fouling, formation of deposits ( preventing or disturbing the heat transfer ). The deposit consists of loose sludge and solid particles. ...
Measuring the Specific Heat Capacity of Water
... Energy for 1˚C temperature rise = total energy used ÷ 15 = _____________ ÷ 15 = ________ J ...
... Energy for 1˚C temperature rise = total energy used ÷ 15 = _____________ ÷ 15 = ________ J ...
Energy Content of Food
... system as follows: A calorie is the amount of energy required to raise 1 gram of water 1 degree Celsius. Click on the food to go to a calorie calculator. ...
... system as follows: A calorie is the amount of energy required to raise 1 gram of water 1 degree Celsius. Click on the food to go to a calorie calculator. ...
living with the lab
... Energy can change form, but it can’t be created or destroyed. Within an isolated system, energy is constant. ...
... Energy can change form, but it can’t be created or destroyed. Within an isolated system, energy is constant. ...
cfd investigation of helical coil heat exchanger abstract
... chemical reactors because they can accommodate a large heat transfer area in a small space, with high heat transfer coefficients. This project deals with the analysis of the helical coiled heat exchanger with various correlations given by different papers for specific conditions. Although various co ...
... chemical reactors because they can accommodate a large heat transfer area in a small space, with high heat transfer coefficients. This project deals with the analysis of the helical coiled heat exchanger with various correlations given by different papers for specific conditions. Although various co ...
Ch. 11 Powerpoint review with answers
... the enthalpy change (in kJ) upon converting 1.00 mol of ice at -25 ° C to water vapor at 125 ° C under constant pressure of 1 atm. The specific heats are ...
... the enthalpy change (in kJ) upon converting 1.00 mol of ice at -25 ° C to water vapor at 125 ° C under constant pressure of 1 atm. The specific heats are ...
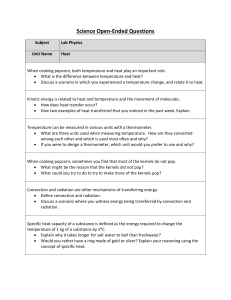
Physics-Heat OEQs
... Specific heat capacity of a substance is defined as the energy required to change the temperature of 1 kg of a substance by 1⁰C. Explain why it takes longer for salt water to boil than freshwater? Would you rather have a ring made of gold or silver? Explain your reasoning using the concept of sp ...
... Specific heat capacity of a substance is defined as the energy required to change the temperature of 1 kg of a substance by 1⁰C. Explain why it takes longer for salt water to boil than freshwater? Would you rather have a ring made of gold or silver? Explain your reasoning using the concept of sp ...
Flat Plate Boundary Layer
... actually touching the tubes would be cooled directly. The amount of heat transferred to the tubes from the fluid running through them depends on the difference in temperature between the tube and the fluid touching it. So if the fluid that is in contact with the tube cools down quickly, less heat wi ...
... actually touching the tubes would be cooled directly. The amount of heat transferred to the tubes from the fluid running through them depends on the difference in temperature between the tube and the fluid touching it. So if the fluid that is in contact with the tube cools down quickly, less heat wi ...
vapor adsorption water cooler using solar thermal energy
... environmental effects. Refrigeration and air conditioning systems powered by solar radiation have an implicit advantage that the peak demand for cooling is in phase with the availability of solar energy. Also, the heat collection arrangement used in these systems can be extended to provide hot water ...
... environmental effects. Refrigeration and air conditioning systems powered by solar radiation have an implicit advantage that the peak demand for cooling is in phase with the availability of solar energy. Also, the heat collection arrangement used in these systems can be extended to provide hot water ...
Heat Transfer Conduction, Convection, and Radiation
... a pool is cooler at the deep end? • Examples: air movement in a home, pot of heating water. • Pick one of these examples and draw the circular pattern in your notes. ...
... a pool is cooler at the deep end? • Examples: air movement in a home, pot of heating water. • Pick one of these examples and draw the circular pattern in your notes. ...
POWERPOINT SCIENCE
... The measure of the average kinetic energy of the individual particles in an object. Matter is made up of tiny particles called atoms and molecules. These particles are always in motion even if the object they make up isn’t moving at all. Energy of motion is called kinetic energy, so all particle ...
... The measure of the average kinetic energy of the individual particles in an object. Matter is made up of tiny particles called atoms and molecules. These particles are always in motion even if the object they make up isn’t moving at all. Energy of motion is called kinetic energy, so all particle ...
Solar water heating

Solar water heating (SWH) is the conversion of sunlight into renewable energy for water heating using a solar thermal collector. Solar water heating systems comprise various technologies that are used worldwide increasingly.In a ""close-coupled"" SWH system the storage tank is horizontally mounted immediately above the solar collectors on the roof. No pumping is required as the hot water naturally rises into the tank through thermosiphon flow. In a ""pump-circulated"" system the storage tank is ground- or floor-mounted and is below the level of the collectors; a circulating pump moves water or heat transfer fluid between the tank and the collectors.SWH systems are designed to deliver hot water for most of the year. However, in winter there sometimes may not be sufficient solar heat gain to deliver sufficient hot water. In this case a gas or electric booster is used to heat the water.