Calorimetry In all physical and chemical reactions energy is either
... In all physical and chemical reactions energy is either released or absorbed. If energy is released in a reaction, it is said to be exothermic and the potential energy of the reactants will be higher than the potential energy of the products. If energy is absorbed in a reaction, it is said to be end ...
... In all physical and chemical reactions energy is either released or absorbed. If energy is released in a reaction, it is said to be exothermic and the potential energy of the reactants will be higher than the potential energy of the products. If energy is absorbed in a reaction, it is said to be end ...
Energy Savings - Boston Heating Supply
... Porcelain Enamel Tank Lining • Engineered for durability and long tank life. ...
... Porcelain Enamel Tank Lining • Engineered for durability and long tank life. ...
Specific Heat Worksheet
... 9. An unknown substance is submerged in a calorimeter of water (an object to help find the specific heat capacity of a substance). The sample is 125 grams, the water Is 150 grams, the water is initially 10 °C and when the sample is submerged, the temperature goes up to 20 °C. What is the specific he ...
... 9. An unknown substance is submerged in a calorimeter of water (an object to help find the specific heat capacity of a substance). The sample is 125 grams, the water Is 150 grams, the water is initially 10 °C and when the sample is submerged, the temperature goes up to 20 °C. What is the specific he ...
Chemistry 2015-2016 Name: Calorimetry Practice Date: Per
... q means heat is released. + q means heat is absorbed. T is always = final temperature – initial temperature. If something is getting hotter (10° 30°) the T is 30 – 10 = + 20°. (heat is absorbed) If something is getting cooler (75° 25°) the T is 25 – 75 = 50°. (heat is released) ...
... q means heat is released. + q means heat is absorbed. T is always = final temperature – initial temperature. If something is getting hotter (10° 30°) the T is 30 – 10 = + 20°. (heat is absorbed) If something is getting cooler (75° 25°) the T is 25 – 75 = 50°. (heat is released) ...
To Measure the Specific Latent heat of Fusion of Ice
... In this IB Lab you will be assessed on the following IB criteria: Data Collection and Evaluation Aim: All heat experiments have problems with heat loss or gain from the surroundings. This experiment contains a trick to try and get round this difficulty. The water is pre-heated to 5 0C above room tem ...
... In this IB Lab you will be assessed on the following IB criteria: Data Collection and Evaluation Aim: All heat experiments have problems with heat loss or gain from the surroundings. This experiment contains a trick to try and get round this difficulty. The water is pre-heated to 5 0C above room tem ...
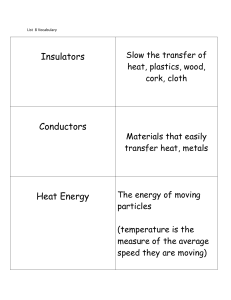
Temperature stabilizing capacity specific heat of water is 1 cal/gram
... • Hydrophilic: polar molecules that dissolve readily in water; sugars, organic acids, some amino acids. • Hydrophobic: non-polar molecules that are not very soluble in water. lipids, some proteins ...
... • Hydrophilic: polar molecules that dissolve readily in water; sugars, organic acids, some amino acids. • Hydrophobic: non-polar molecules that are not very soluble in water. lipids, some proteins ...
Notes - hrsbstaff.ednet.ns.ca
... A sample of mercury (c = 0.14 J/goC) is heated from 25.5oC to 52.5oC. In the process 3050J of heat are absorbed. What mass of mercury was contained in the sample? ...
... A sample of mercury (c = 0.14 J/goC) is heated from 25.5oC to 52.5oC. In the process 3050J of heat are absorbed. What mass of mercury was contained in the sample? ...
Snow-melting and Deicing System Using Underground Thermal
... (1) Use of stable, clean geothermal energy as a heat source The system utilizes natural geothermal energy. Therefore, this abundant natural resource offers a sufficient and stable quantities of heat even during the cold winter months. In addition, the system employs a high-performance ground source ...
... (1) Use of stable, clean geothermal energy as a heat source The system utilizes natural geothermal energy. Therefore, this abundant natural resource offers a sufficient and stable quantities of heat even during the cold winter months. In addition, the system employs a high-performance ground source ...
Heat Transfer Comparison in Coaxial Tube in Tube Heat Exchanger
... exchange of those by new, ecologically acceptable, HFC refrigerants. Therefore system performance analyses was made where the single component refrigerant R22 was replaced with zeotropic mixture R407C. In the system operating at the same conditions, a comparison of heat transfer in coaxial exchanger ...
... exchange of those by new, ecologically acceptable, HFC refrigerants. Therefore system performance analyses was made where the single component refrigerant R22 was replaced with zeotropic mixture R407C. In the system operating at the same conditions, a comparison of heat transfer in coaxial exchanger ...
Specific Heat of Metals Make Up Directions
... to run. Part 1 is Iron and part 2 is Copper. 2. Use the mass of the water and the metal from your data table below. 3. Using the thermometer on the screen, record the initial temperature of the water under Water A on the data table below before placing the metal in the water. The initial temperature ...
... to run. Part 1 is Iron and part 2 is Copper. 2. Use the mass of the water and the metal from your data table below. 3. Using the thermometer on the screen, record the initial temperature of the water under Water A on the data table below before placing the metal in the water. The initial temperature ...
Note: Moving air
... Question: Describe how conduction, convection, and radiation play a role in losing heat through a double-pane window. Answer: Heat is conducted through the solid glass and through the four air films, two on each side of each sheet of glass. The air films do the bulk of the insulating! If the space b ...
... Question: Describe how conduction, convection, and radiation play a role in losing heat through a double-pane window. Answer: Heat is conducted through the solid glass and through the four air films, two on each side of each sheet of glass. The air films do the bulk of the insulating! If the space b ...
Page 45a of James Watt`s Laboratory Notebook
... subtract heat given by cone 143 x 72 = 10296 ÷ by 2/3 ...
... subtract heat given by cone 143 x 72 = 10296 ÷ by 2/3 ...
Physical Property Notes
... Some things heat up and cool down fast…like sand on the beach in the summer time These substances have ____________ specific heat Some things heat up and cool down slowly…like water in the ocean in the summer time These substances have ____________ specific heat Calculations ...
... Some things heat up and cool down fast…like sand on the beach in the summer time These substances have ____________ specific heat Some things heat up and cool down slowly…like water in the ocean in the summer time These substances have ____________ specific heat Calculations ...
Document
... The number of BTU’s (British Thermal Units) per hour that will flow through 1 sq. ft. of the structure when there is a 1 degree difference in temperature between the 2 sides. The higher the U-value; the more heat will be lost ...
... The number of BTU’s (British Thermal Units) per hour that will flow through 1 sq. ft. of the structure when there is a 1 degree difference in temperature between the 2 sides. The higher the U-value; the more heat will be lost ...
Chapter 6 Lesson 2 Name_____________ Describe the three ways
... In Figure 6-1, most of the heat provided by the fireplace in room C goes up the chimney and is therefore transferred by ____________________. In Figure 6-1, the thermal energy of the iron in room D is transferred to the clothes by ____________________. In Figure 6-1, room A, the heat from the pot on ...
... In Figure 6-1, most of the heat provided by the fireplace in room C goes up the chimney and is therefore transferred by ____________________. In Figure 6-1, the thermal energy of the iron in room D is transferred to the clothes by ____________________. In Figure 6-1, room A, the heat from the pot on ...
Solar water heating

Solar water heating (SWH) is the conversion of sunlight into renewable energy for water heating using a solar thermal collector. Solar water heating systems comprise various technologies that are used worldwide increasingly.In a ""close-coupled"" SWH system the storage tank is horizontally mounted immediately above the solar collectors on the roof. No pumping is required as the hot water naturally rises into the tank through thermosiphon flow. In a ""pump-circulated"" system the storage tank is ground- or floor-mounted and is below the level of the collectors; a circulating pump moves water or heat transfer fluid between the tank and the collectors.SWH systems are designed to deliver hot water for most of the year. However, in winter there sometimes may not be sufficient solar heat gain to deliver sufficient hot water. In this case a gas or electric booster is used to heat the water.