CHAPTER-3 CLASSIFICATION OF ELEMENTS
... added, the number of electrons goes up by one. This results in an increase in repulsion among the electrons. However, the number of protons remains the same. As a result, the effective nuclear charge of the atom decreases and the radius of the atom increases. (b) When an atom loses an electron, the ...
... added, the number of electrons goes up by one. This results in an increase in repulsion among the electrons. However, the number of protons remains the same. As a result, the effective nuclear charge of the atom decreases and the radius of the atom increases. (b) When an atom loses an electron, the ...
Chem Ch 5 Release Test
... d. Moseley The most useful source of general information about the elements for anyone associated with chemistry is a a. calculator. c. periodic table. b. table of metric equivalents. d. table of isotopes. The periodic table a. permits the properties of an element to be predicted before the element ...
... d. Moseley The most useful source of general information about the elements for anyone associated with chemistry is a a. calculator. c. periodic table. b. table of metric equivalents. d. table of isotopes. The periodic table a. permits the properties of an element to be predicted before the element ...
chemistry - Illini West High School
... called the lanthanide series and actinide series and are located at the bottom of the periodic table. ...
... called the lanthanide series and actinide series and are located at the bottom of the periodic table. ...
11-chemistry-exemplar-chapter-3
... Each question has one correct option. Choose the correct option. In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic t ...
... Each question has one correct option. Choose the correct option. In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic t ...
H Unit 4: Periodic Table
... The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. There are a number of major groups with similar properties. They are as follows: ...
... The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. There are a number of major groups with similar properties. They are as follows: ...
Periodic Table Element Pattern
... arranges elements into vertical columns (Groups) and horizontal rows (Periods) to display these commonalities. ...
... arranges elements into vertical columns (Groups) and horizontal rows (Periods) to display these commonalities. ...
File
... The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. There are a number of major groups with similar properties. They are as follows: ...
... The table is also arranged in vertical columns called “groups” or “families” and horizontal rows called “periods.” Each arrangement is significant. The elements in each vertical column or group have similar properties. There are a number of major groups with similar properties. They are as follows: ...
Science 2nd prep 1st term 1st lesson Many attempts are made by
... Description of the periodic table *The number of known elements until now are 116 elements, 92 elements are available in the earth’s crust, the rest of the elements are prepared artificially. *Elements of (A) groups lie on the left and right of the table, you can locate their position in the modern ...
... Description of the periodic table *The number of known elements until now are 116 elements, 92 elements are available in the earth’s crust, the rest of the elements are prepared artificially. *Elements of (A) groups lie on the left and right of the table, you can locate their position in the modern ...
The physical characteristics of the atom of an element are called
... 15. The atomic radii of noble gases are not considered here. Being monatomic, their (non-bonded radii) values are very large. In fact radii of noble gases should be compared not with the covalent radii but with the van der Waals radii of other elements. 16. A cation is smaller than its parent atom b ...
... 15. The atomic radii of noble gases are not considered here. Being monatomic, their (non-bonded radii) values are very large. In fact radii of noble gases should be compared not with the covalent radii but with the van der Waals radii of other elements. 16. A cation is smaller than its parent atom b ...
Periodic Trends Studyguide with Questions and Answers
... MY2 is the general formula of a Group 2 atom bonding with a Group 17 halogen (Y) MO is the general formula of a Group 2 atom bonding with O (to form an oxide) . Radium (Ra) is the most reactive metal in this group. Radium is also radioactive. . All alkaline earth metals exist as solids at STP ...
... MY2 is the general formula of a Group 2 atom bonding with a Group 17 halogen (Y) MO is the general formula of a Group 2 atom bonding with O (to form an oxide) . Radium (Ra) is the most reactive metal in this group. Radium is also radioactive. . All alkaline earth metals exist as solids at STP ...
The Periodic Table
... USING THE PERIODIC TABLE Rows are called periods. Columns are called groups. ...
... USING THE PERIODIC TABLE Rows are called periods. Columns are called groups. ...
Periodic Properties of the Elements
... 1. In Exploration 1, select the element from period 2, group 1, on the periodic table. When selected, the group and period number will be highlighted by red circles. 2. Select the element’s period (row) number, represented by a red circle. 3. Record the element’s properties from the list provided in ...
... 1. In Exploration 1, select the element from period 2, group 1, on the periodic table. When selected, the group and period number will be highlighted by red circles. 2. Select the element’s period (row) number, represented by a red circle. 3. Record the element’s properties from the list provided in ...
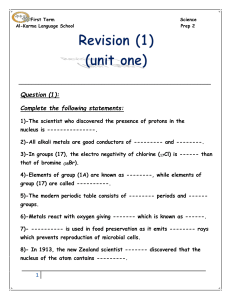
First Term Science Al-Karma Language School Prep 2 Question (1
... 12)-Sodium and potassium are kept under the surface of --------- to prevent them from the reaction with ----------. 13)-Each period in the modern periodic table starts with ------- and ends with ---------. 14)-The positive ion carries a number of ---------- charges equals to the number -------- elec ...
... 12)-Sodium and potassium are kept under the surface of --------- to prevent them from the reaction with ----------. 13)-Each period in the modern periodic table starts with ------- and ends with ---------. 14)-The positive ion carries a number of ---------- charges equals to the number -------- elec ...
The Periodic Table
... at regular intervals —like the appearance of Haley’s comet every 76 years ...
... at regular intervals —like the appearance of Haley’s comet every 76 years ...
Periodic Table Oakland Schools Chemistry Resource Unit Andrew D. Hulbert
... Mendeleev started a new row each time he noticed that the chemical properties of the elements repeated. He placed elements in the new row directly below elements of similar chemical properties in the preceding row. Amazingly, Mendeleev predicted the properties of the missing elements in his table, l ...
... Mendeleev started a new row each time he noticed that the chemical properties of the elements repeated. He placed elements in the new row directly below elements of similar chemical properties in the preceding row. Amazingly, Mendeleev predicted the properties of the missing elements in his table, l ...
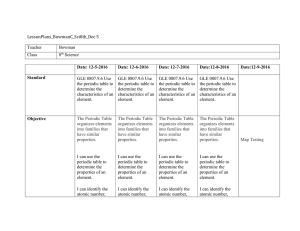
LessonPlans_BowmanC_Sci8th_Dec 5 Teacher Bowman Class 8th
... atomic mass, number of protons, neutrons, and electrons in an atom of an element using the periodic table. ...
... atomic mass, number of protons, neutrons, and electrons in an atom of an element using the periodic table. ...
Chemical Elements Essay Research Paper At first
... question began to emerge. Are the chemical elements really as different as they seem? Or is there some underlying principle that can be used to organize the apparent complexity that exists? ORGANIZING THE ELEMENTS By the mid-nineteenth century, chemists had discovered more than sixty elements. New e ...
... question began to emerge. Are the chemical elements really as different as they seem? Or is there some underlying principle that can be used to organize the apparent complexity that exists? ORGANIZING THE ELEMENTS By the mid-nineteenth century, chemists had discovered more than sixty elements. New e ...
unit 3 ppt
... For energy level n, there are n possible sublevels, so the d sublevel first appears when n=3. This 3d sublevel is slightly higher in energy than the 4s sublevel, so these are filled in the order 4s3d.This order of filling is also seen for higher values of n. Each d sublevel consists of five orbitals ...
... For energy level n, there are n possible sublevels, so the d sublevel first appears when n=3. This 3d sublevel is slightly higher in energy than the 4s sublevel, so these are filled in the order 4s3d.This order of filling is also seen for higher values of n. Each d sublevel consists of five orbitals ...
periodic classification of elements
... electrons are placed in one group. Outer shell electrons are identical in each group. The elements within a group show: (i) Similar properties due to similar electronic configuration. (ii) A gradation of properties due to slowly varying attraction between nucleus and the valence electrons. (iii) Acc ...
... electrons are placed in one group. Outer shell electrons are identical in each group. The elements within a group show: (i) Similar properties due to similar electronic configuration. (ii) A gradation of properties due to slowly varying attraction between nucleus and the valence electrons. (iii) Acc ...
Lesson 7.8 Basic Properties of the Main Group Elements Suggested
... YouTube Video We have already mentioned the distinct trend from nonmetal to metal going down the Group IVA elements. Carbon Exists in two well-known allotropic forms: graphite, a soft, black substance used in pencil leads and diamond, a very hard, clear, crystalline substance. Another allotrope a ca ...
... YouTube Video We have already mentioned the distinct trend from nonmetal to metal going down the Group IVA elements. Carbon Exists in two well-known allotropic forms: graphite, a soft, black substance used in pencil leads and diamond, a very hard, clear, crystalline substance. Another allotrope a ca ...
Periodic Table of Elements
... elements are organized based on their atomic numbers, electron configurations, and recurring chemical properties. In the periodic table, elements are presented in order of increasing atomic number (the number of protons). The rows of the table are called periods; the columns of the s- (columns 1-2 a ...
... elements are organized based on their atomic numbers, electron configurations, and recurring chemical properties. In the periodic table, elements are presented in order of increasing atomic number (the number of protons). The rows of the table are called periods; the columns of the s- (columns 1-2 a ...
Periodicity of Elements and Periodic Table CHAPTER – 4
... Properties of Group VII 1. They are diatomic except At. 2. Halogens are very active non-metals. 3. Outer most shell of these elements is incomplete having seven electrons. 4. Elements of this group are highly reactive. 5. There is a gradual decrease in the ionization potential down the group. Transi ...
... Properties of Group VII 1. They are diatomic except At. 2. Halogens are very active non-metals. 3. Outer most shell of these elements is incomplete having seven electrons. 4. Elements of this group are highly reactive. 5. There is a gradual decrease in the ionization potential down the group. Transi ...
Periods and Blocks of the Periodic Table
... • In many compounds, the negative charge of the valence electrons is concentrated closer to one atom than to another. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. • Electronegativities tend to increase acros ...
... • In many compounds, the negative charge of the valence electrons is concentrated closer to one atom than to another. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. • Electronegativities tend to increase acros ...
Periodic Table
... f-block Inner Transition Metals For period n (n = 6 or 7) • Filled ns (ns2); Filled or partially filled (n-2) f (4f ...
... f-block Inner Transition Metals For period n (n = 6 or 7) • Filled ns (ns2); Filled or partially filled (n-2) f (4f ...